Publications
Original Papers
- K. Oga, Y. Yamada, M. Nagatomo, H. Fujino, M. Inoue, “Collective Total Synthesis of 12 C4-Oxygenated Cladiellins and Structure Elucidation of Cladieunicellin D and Cladielloides A/C,”
J. Am. Chem. Soc. 2026, 148, 1812–1823.
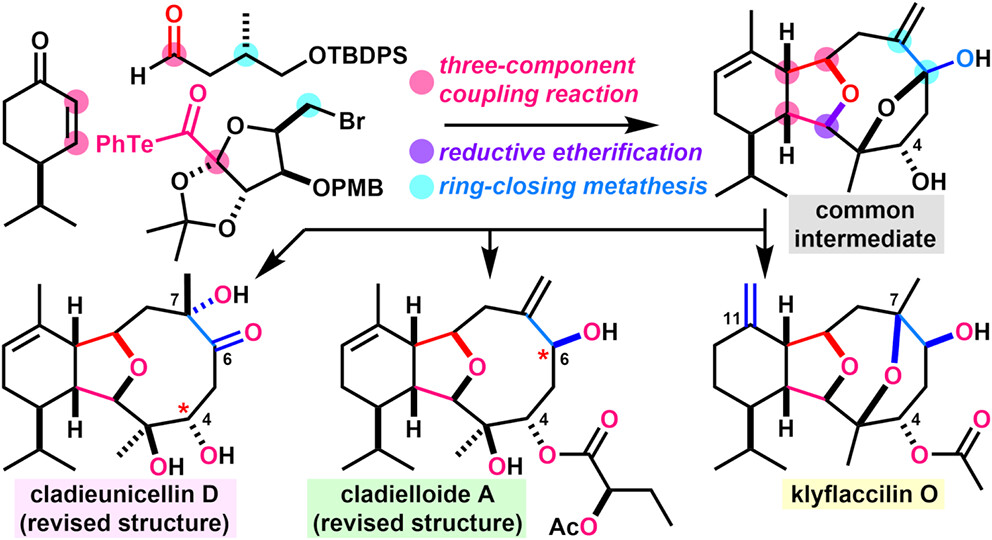
DOI: 10.1021/jacs.5c19112
- K. Takaoka, D. Matsubara, M. Matsumoto, M. Nagatomo, K. Hagiwara, M. Inoue, “Total Synthesis of Trigocherrins A and C,”
J. Am. Chem. Soc. 2025, 147, 45670–45679.
(Selected as Most Read Articles)
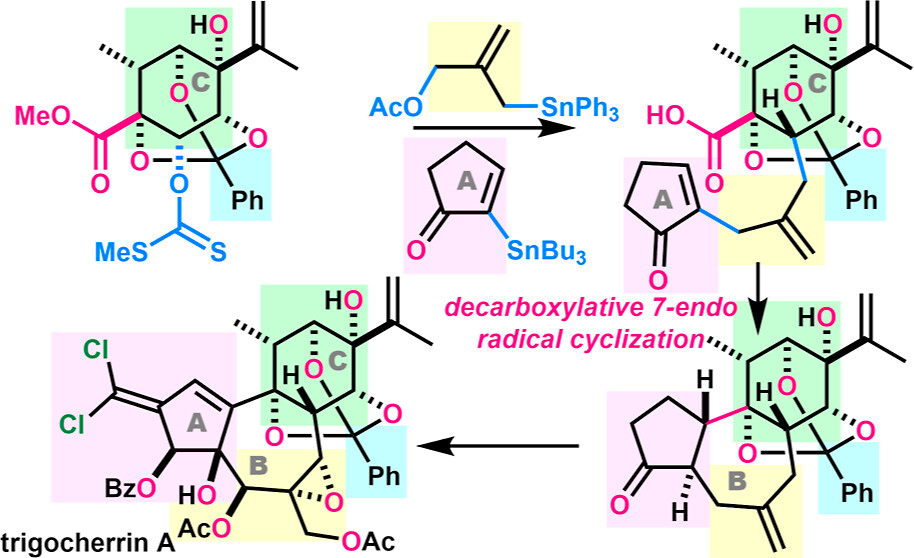
DOI: 10.1021/jacs.5c17272
- Y. Yamada, R. Yoshinaga, Y. Matsui, M. Nagatomo, H. Fujino, M. Inoue, “Et3Al/Light-Promoted Radical-Polar Crossover Reactions of α-Alkoxyacyl Tellurides” J. Org. Chem. 2024, 89, 11701–11706.
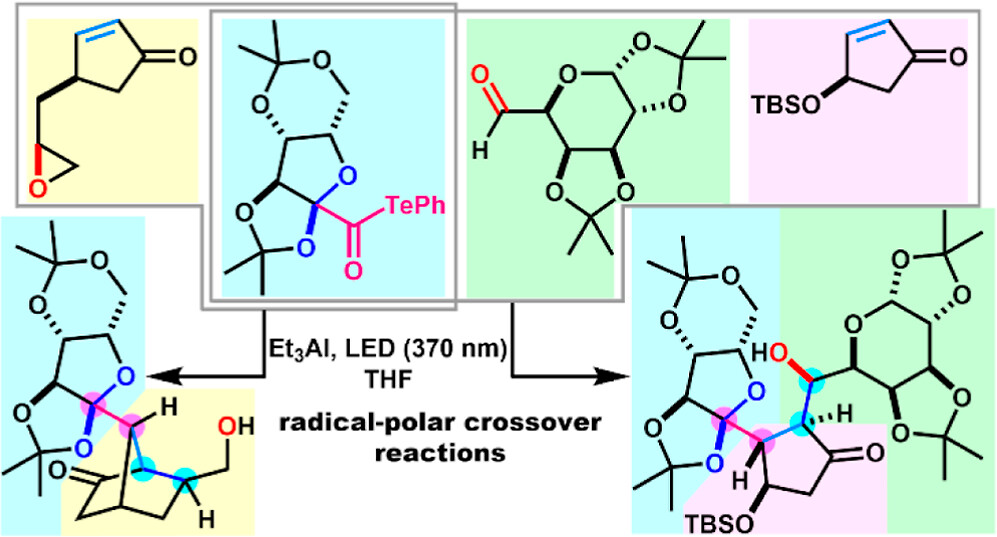
DOI: 10.1021/acs.joc.4c01445
- A. Watanabe, Y. Hikone, M. Nagatomo, M. Inoue, “Conversion of Phorbol into Des-D-Ring Tricycle and Crotonianoid B via Peroxidation Reaction,” Org. Lett. 2024, 26, 4335–4339.
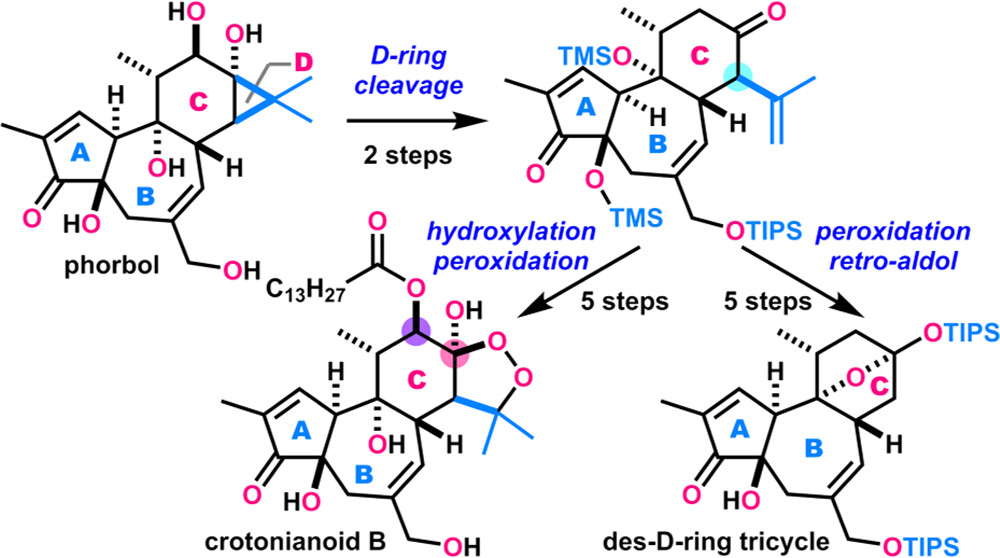
DOI: 10.1021/acs.orglett.4c01363
- A. Watanabe, M. Nagatomo, A. Hirose, Y. Hikone, N. Kishimoto, S. Miura, T. Yasutake, T. Abe, S. Misumi, M. Inoue, “Total Syntheses of Phorbol and 11 Tigliane Diterpenoids and Their Evaluation as HIV Latency-Reversing Agents,” J. Am. Chem. Soc. 2024, 146, 8746–8756.
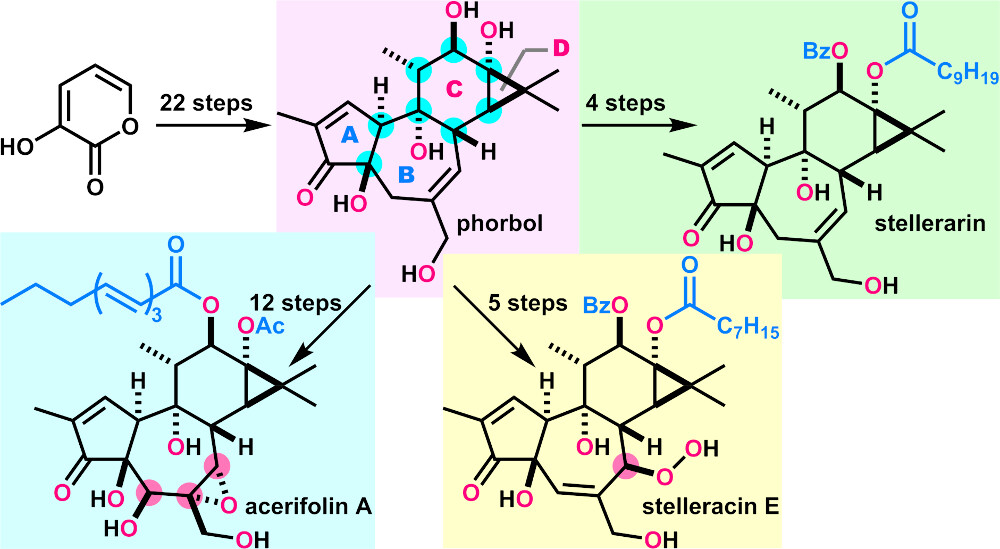
DOI: 10.1021/jacs.4c01589
- T. Watanabe, K. Oga, H. Matoba, M. Nagatomo, M. Inoue, “Total Synthesis of Taxol Enabled by Intermolecular Radical Coupling and Pd-Catalyzed Cyclization,” J. Am. Chem. Soc. 2023, 145, 25894–25902.
(23/12/4 Selected as Most Read Articles)
(24/1/9 Featured in “Some Items of Interest to Process R&D Chemists and Engineers” Org. Process Res. Dev. 2024, 28, 1.)
(Featured in Synfacts)
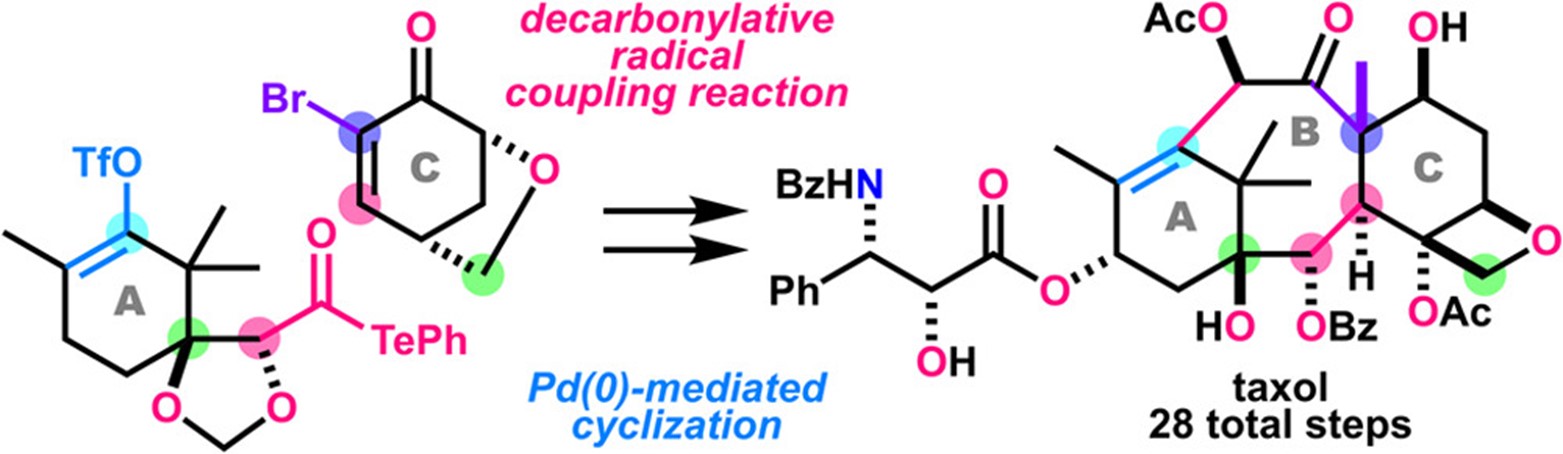
DOI: 10.1021/jacs.3c10658
- Y. Imamura, K. Takaoka, Y. Komori, M. Nagatomo, M. Inoue, “Total Synthesis of Taxol Enabled by Inter- and Intramolecular Radical Coupling Reactions,” Angew. Chem. Int. Ed. 2023, 62, e202219114.
(Selected as Hot Paper)
(Featured in Synfacts)
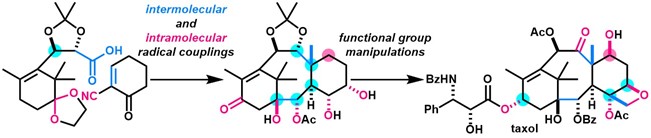
DOI: 10.1002/anie.202219114
- Y. Hikone, T. Kato, M. Nagatomo, M. Inoue, “Total Synthesis of Resiniferatoxin Enabled by Photocatalytic Decarboxylative Radical Cyclization,” Org. Lett. 2022, 24, 929–933.
(Selected as Most Read Articles)
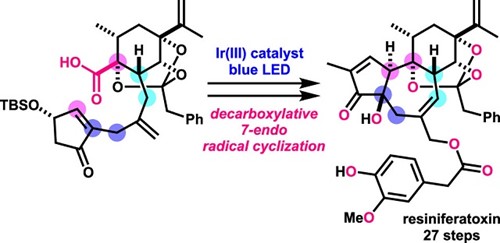
DOI: 10.1021/acs.orglett.1c04286
- D. Kuwana, Y. Komori, M. Nagatomo, M. Inoue, “Photoinduced Decarboxylative Radical Coupling Reaction of Multiply Oxygenated Structures by Catalysis of Pt-Doped TiO2,” J. Org. Chem. 2022, 87, 730–736.
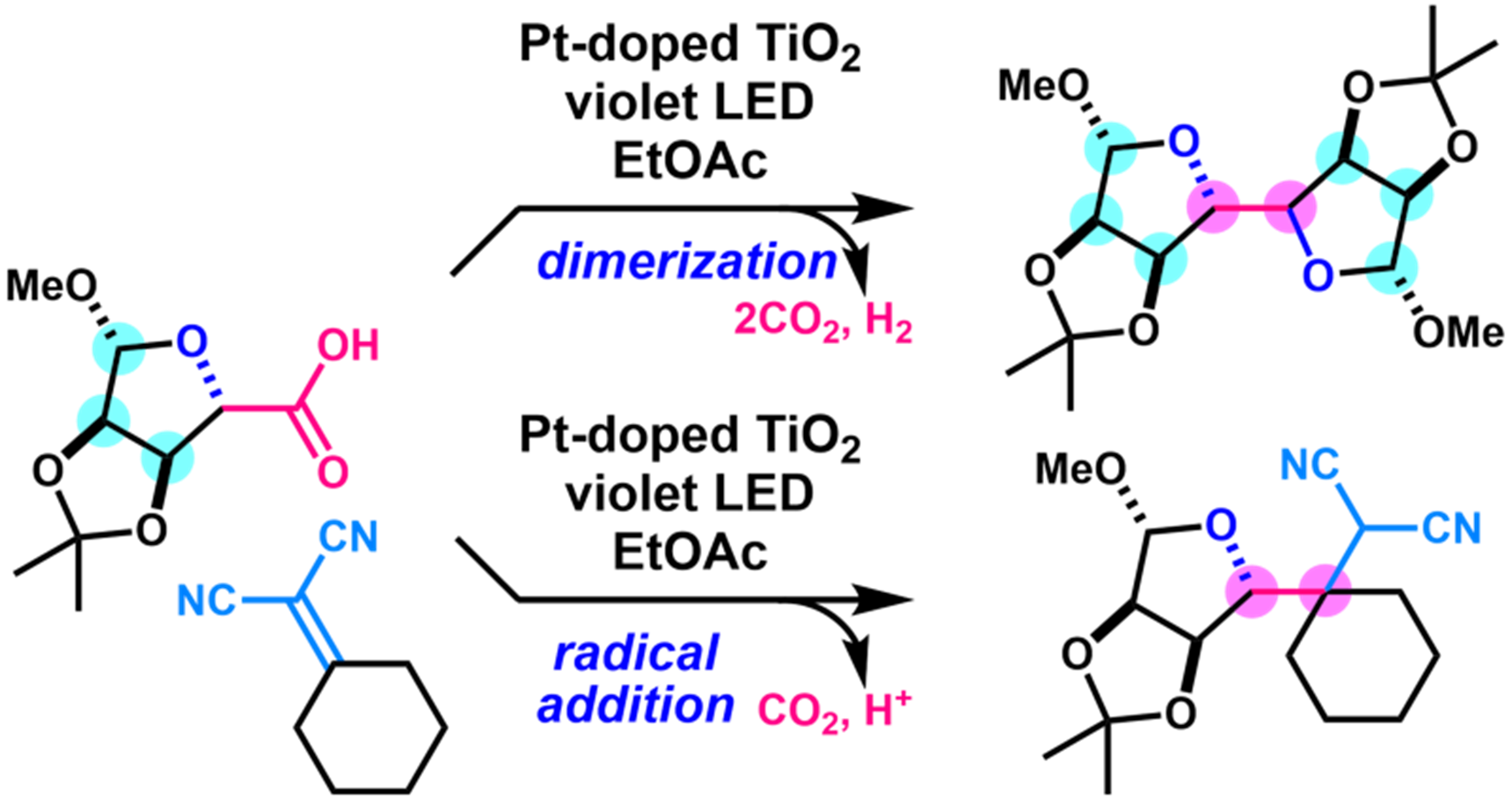
DOI: 10.1021/acs.joc.1c02736
- A. Hirose, A. Watanabe, K. Ogino, M. Nagatomo, M. Inoue, “Unified Total Syntheses of Rhamnofolane, Tigliane, and Daphnane Diterpenoids,” J. Am. Chem. Soc. 2021, 143, 12387–12396.
(Featured in Synfacts)
(Highlighted in Organic Chemistry Highlights)
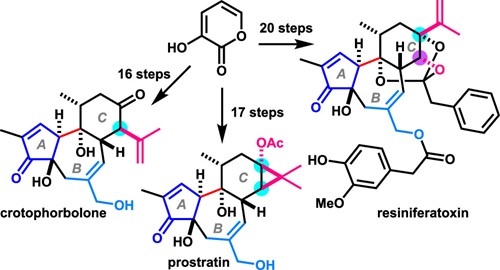
DOI: 10.1021/jacs.1c06450
- M. Nagatomo, K. Zhang, H. Fujino, M. Inoue, “Et3B/Et2AlCl/O2‐Mediated Radical Coupling Reaction between α‐Alkoxyacyl Tellurides and 2‐Hydroxybenzaldehyde Derivatives,” Chem. Asian. J. 2020, 15, 3820–3824.
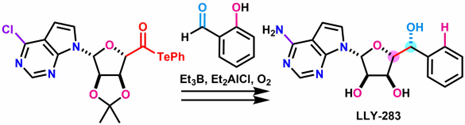
DOI: 10.1002/asia.202001090
- T. Fukuda, M. Nagatomo, M. Inoue, “Total Synthesis of Diospyrodin and Its Three Diastereomers,” Org. Lett. 2020, 22, 6468–6472.
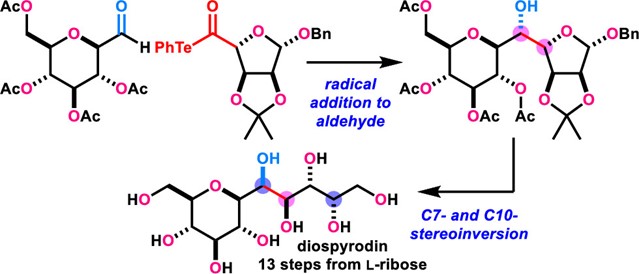
DOI: 10.1021/acs.orglett.0c02280
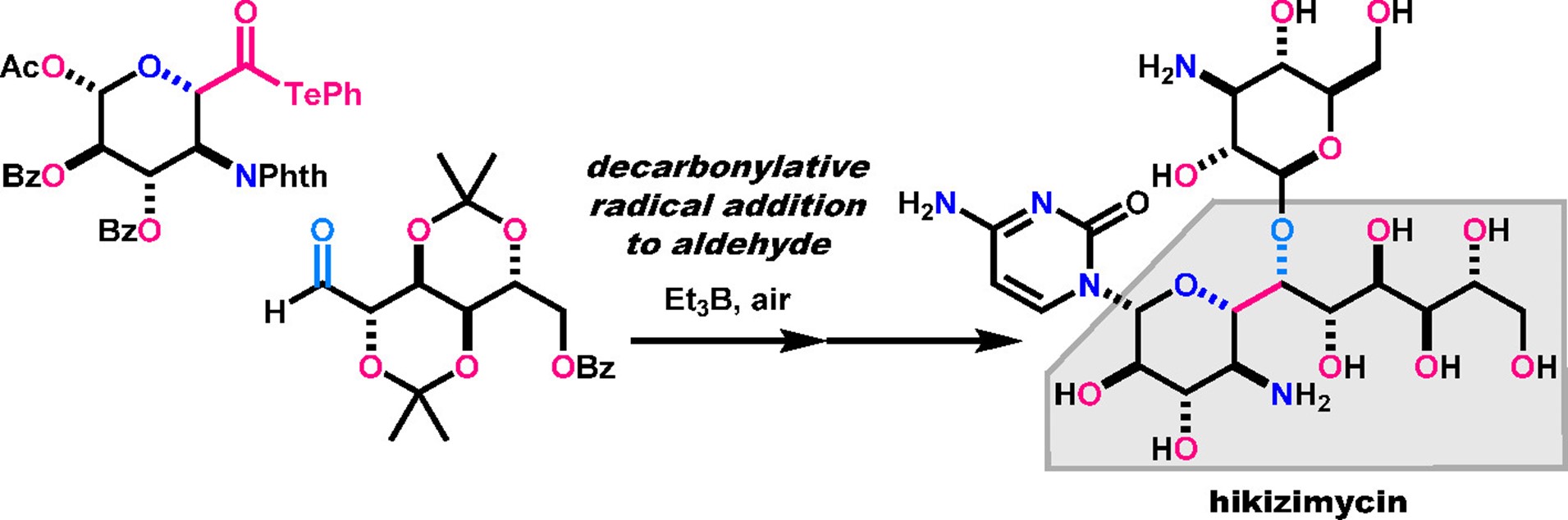
- H. Fujino, T. Fukuda, M. Nagatomo, M. Inoue, “Convergent Total Synthesis of Hikizimycin Enabled by Intermolecular Radical Addition to Aldehyde,” J. Am. Chem. Soc. 2020, 142, 13227–13234.
(Highlighted in Organic Chemistry Highlights)
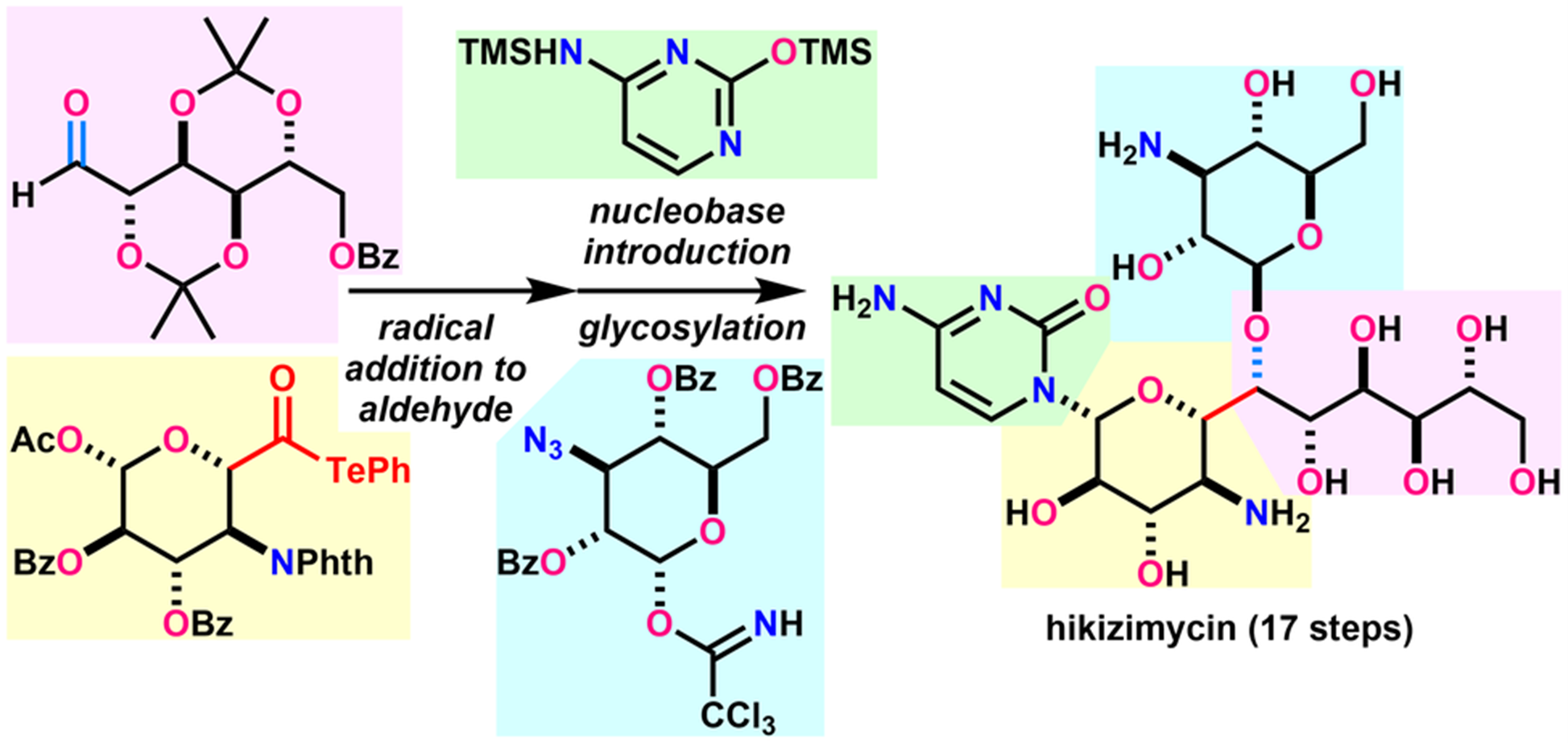
DOI: 10.1021/jacs.0c06354
- D. Kuwana, M. Nagatomo, M. Inoue, “Total Synthesis of 5-epi-Eudesm-4(15)-ene-1β,6β-diol via Decarbonylative Radical Coupling Reaction,” Org. Lett. 2019, 21, 7619–7623.
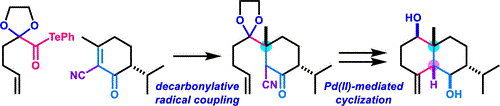
DOI: 10.1021/acs.orglett.9b02895
- Y. Imamura, S. Yoshioka, M. Nagatomo, M. Inoue, “Total Synthesis of 1‐Hydroxytaxinine,” Angew. Chem. Int. Ed. 2019, 58, 12159–12163.
(Selected as Hot Paper)
(Highlighted in Organic Chemistry Highlights)
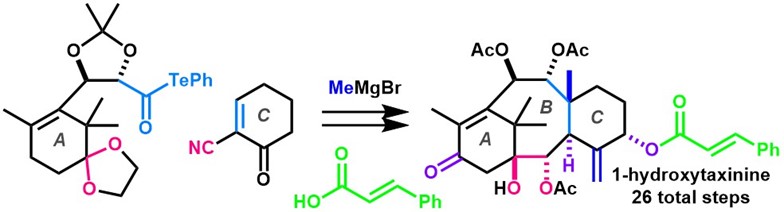
DOI: 10.1002/anie.201906872
- D. Kuwana, B. Ovadia, D. Kamimura, M. Nagatomo, M. Inoue, “Installation of O‐Heterocycles to N‐Heteroarenes via an Et3B/O2‐mediated Radical Reaction of α-Alkoxy and α-Alkoxyacyl Tellurides,” Asian J. Org. Chem. 2019, 8, 1088–1091.
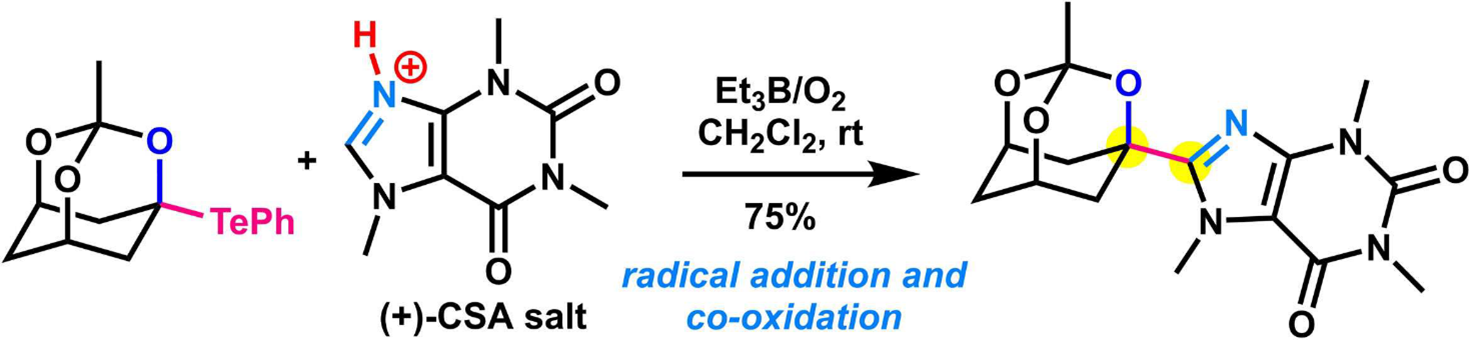
DOI: 10.1002/ajoc.201900170
- M. Nagatomo, Y. Fujimoto, K. Masuda, M. Inoue, “Construction of a 6/5/9-membered tricyclic structure of cladiellins via radical-polar crossover reaction,” J. Antibiot. 2019, 72, 486–489.
DOI: 10.1038/s41429-019-0150-7
- H. Matoba, T. Watanabe, M. Nagatomo, M. Inoue, “Convergent Synthesis of Taxol Skeleton via Decarbonylative Radical Coupling Reaction,” Org. Lett. 2018, 20, 7554–7557.
(Selected as Most Read Articles of the month)
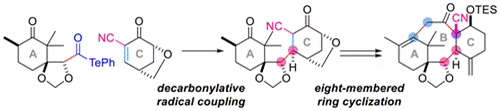
DOI: 10.1021/acs.orglett.8b03302
- T. Kawamata, A. Yamaguchi, M. Nagatomo, M. Inoue, “Convergent Total Synthesis of Asimicin via Decarbonylative Radical Dimerization,” Chem. Eur. J. 2018, 24, 18907–18912.
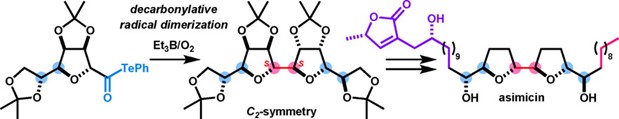
DOI: 10.1002/chem.201805317
- D. Urabe, Y. Nakagawa, K. Mukai, K. Fukushima, N. Aoki, H. Itoh, M. Nagatomo, M. Inoue, “Total synthesis and biological evaluation of 19-hydroxysarmentogenin-3-O-a-L-rhamnoside, trewianin, and their aglycons,” J. Org. Chem. 2018, 83, 13888–13910.
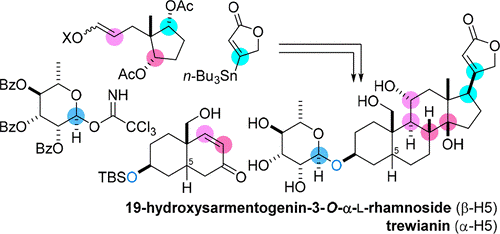
DOI: 10.1021/acs.joc.8b02219
- M. Koshimizu, M. Nagatomo, M. Inoue, “Construction of a pentacyclic ring system of isoryanodane diterpenoids by SmI2-mediated transannular cyclization,” Tetrahedron. 2018, 74, 3384–3390.
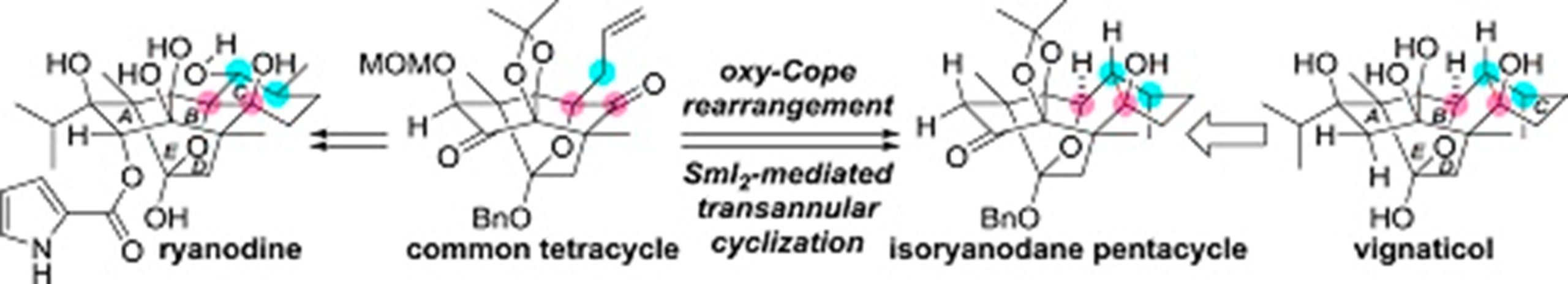
DOI: 10.1016/j.tet.2018.03.061
- H. Fujino, M. Nagatomo, A. Paudel, S. Panthee, H. Hamamoto, K. Sekimizu, M. Inoue, “Unified Total Synthesis of Polyoxin J, L, and Their Fluorinated Analogues on the Basis of Decarbonylative Radical Coupling Reactions,” Angew. Chem. Int. Ed. 2017, 56, 11865–11869.
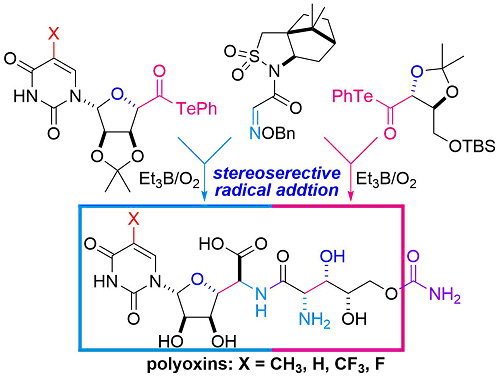
DOI: 10.1002/anie.201706671
- K. Masuda, M. Tanigawa, M. Nagatomo, D. Urabe, M. Inoue, “Construction of carbocycles initiated by Cu-catalyzed radical reaction of Cl2C(CN)2,” Tetrahedron 2017, 73, 3596–3605.
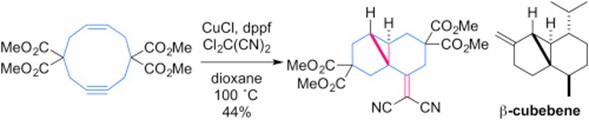
DOI: 10.1016/j.tet.2017.03.063
- T. Kawamata, M. Nagatomo, M. Inoue, “Total Synthesis of Zaragozic Acid C: Implementation of Photochemical C(sp3)-H Acylation,” J. Am. Chem. Soc. 2017, 139, 1814–1817.
(Highlighted in Synfacts 2017, 13, 0337. and selected as its Cover Picture)
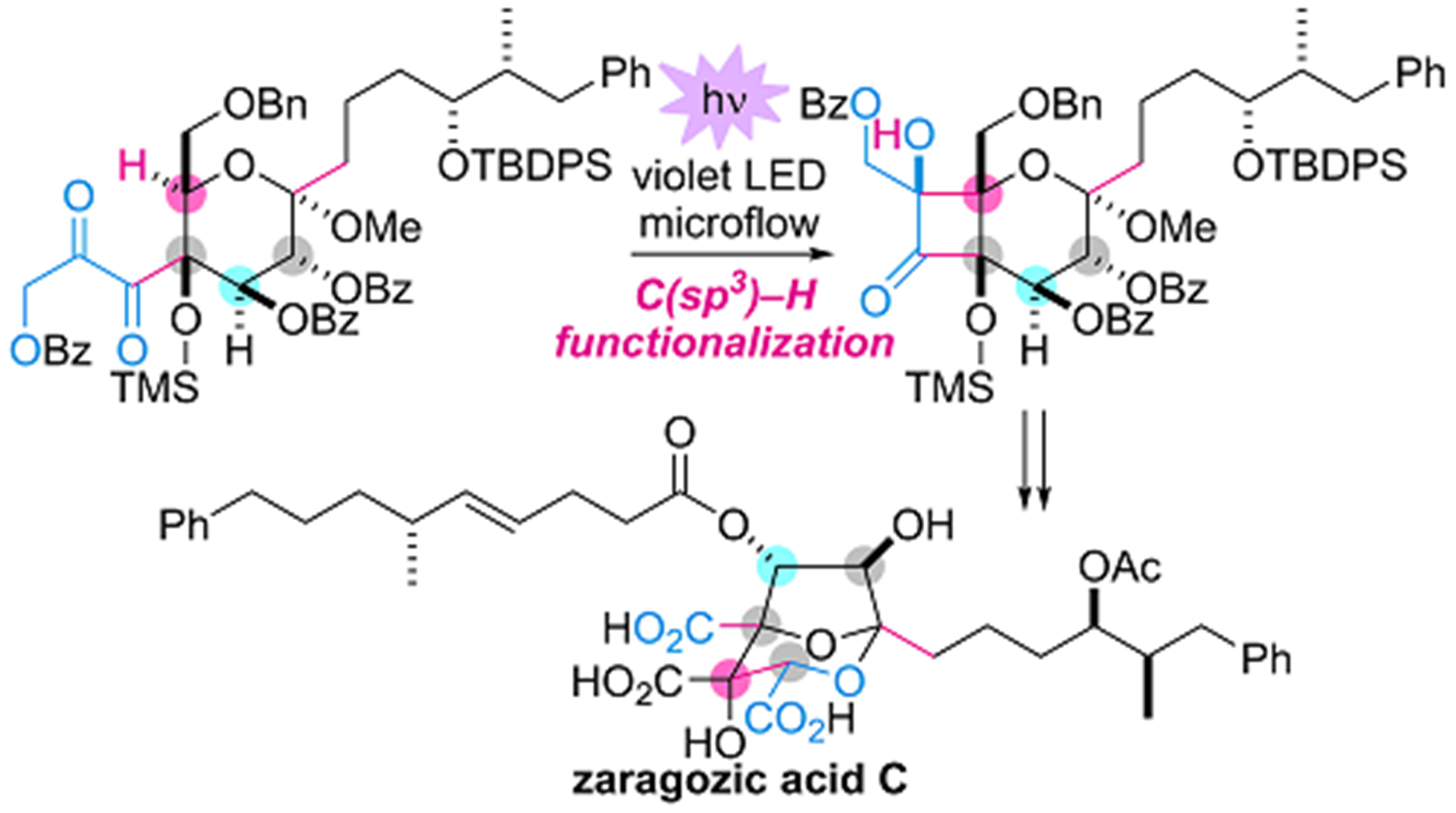
DOI: 10.1021/jacs.6b13263
- K. Masuda, M. Nagatomo, M. Inoue, “Direct assembly of multiply oxygenated carbon chains by decarbonylative radical-radical coupling reactions,” Nature Chem. 2017, 9, 207–212.
(Highlighted in Synfacts 2017, 13, 0534. )
(Associated Link: News and Views – “Organic chemistry: A radical step forward”)
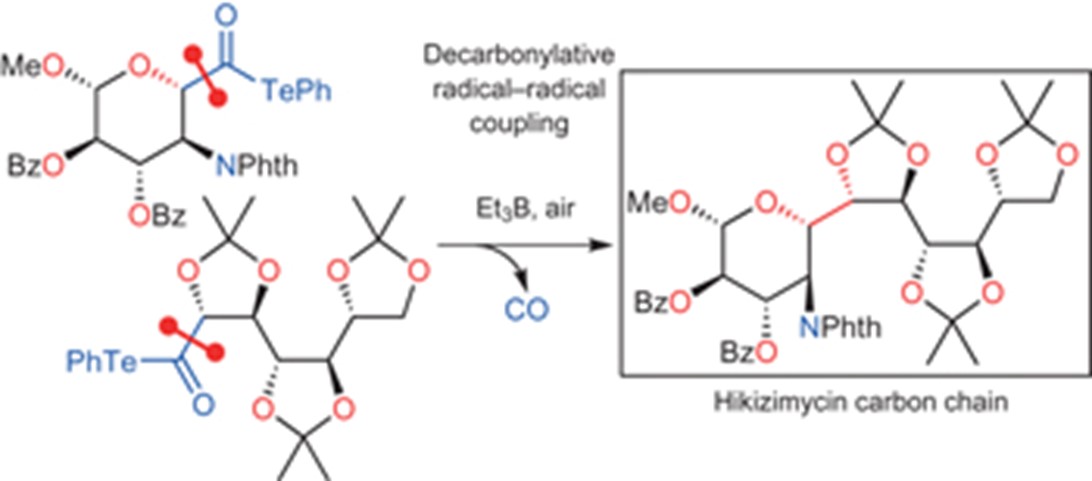
DOI: 10.1038/nchem.2639
- S. Matsumura, Y. Matsui, M. Nagatomo, M. Inoue, “Stereoselective construction of anti– and syn-1,2-diol structures via decarbonylative radical coupling of α-alkoxyacyl tellurides,” Tetrahedron 2016, 72, 4859–4866.
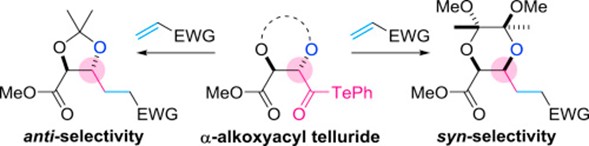
DOI: 10.1016/j.tet.2016.06.056
- D. Kamimura, M. Nagatomo, D. Urabe, M. Inoue, “Expanding the scope of Et3B/O2-mediated coupling reactions of O,Te-acetal,” Tetrahedron 2016, 72, 7839–7848.
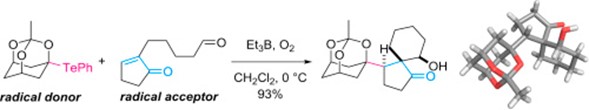
DOI: 10.1016/j.tet.2016.04.023
- K. Masuda, M. Nagatomo, M. Inoue, “Chemical Conversion of Ryanodol to Ryanodine,” Chem. Pharm. Bull. 2016, 64, 874–879.
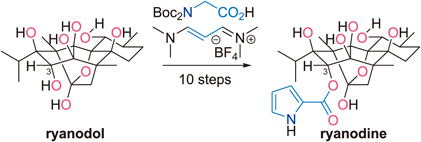
DOI: 10.1248/cpb.c16-00214
- M. Koshimizu, M. Nagatomo, M. Inoue, “Unified Total Synthesis of 3-epi-Ryanodol, Cinnzeylanol, Cinncassiols A and B, and Structural Revision of Natural Ryanodol and Cinnacasol,” Angew. Chem. Int. Ed. 2016, 55, 2493–2497.
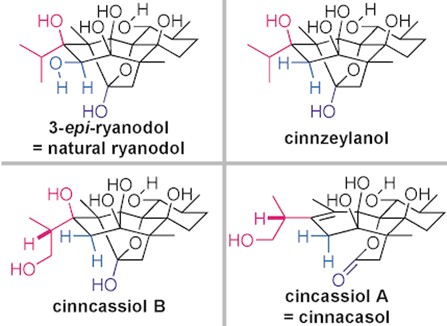
DOI: 10.1002/anie.201511116
- K. Masuda, M. Koshimizu, M. Nagatomo, M. Inoue, “Asymmetric Total Synthesis of (+)-Ryanodol and (+)-Ryanodine,” Chem. Eur. J. 2016, 22, 230–236.
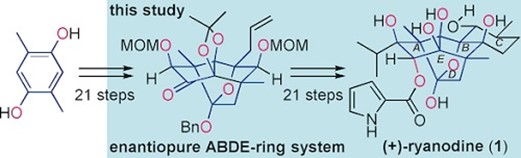
DOI: 10.1002/chem.201503641
- M. Nagatomo, K. Hagiwara, K. Masuda, M. Koshimizu, T. Kawamata, Y. Matsui, D. Urabe, M. Inoue, ” Symmetry-Driven Strategy for the Assembly of the Core Tetracycle of (+)-Ryanodine: Synthetic Utility of a Cobalt-Catalyzed Olefin Oxidation and α-Alkoxy Bridgehead Radical Reaction,” Chem. Eur. J. 2016, 22, 222–229.
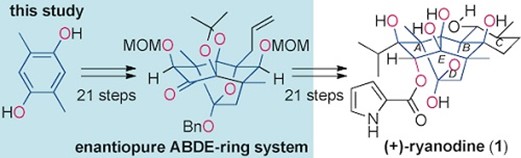
DOI: 10.1002/chem.201503640
- M. Nagatomo, D. Kamimura, Y. Matsui, K. Masuda, M. Inoue, “Et3B-Mediated Two- and Three-Component Coupling Reactions via Radical Decarbonylation of α-Alkoxyacyl Tellurides: Single-Step Construction of Densely Oxygenated Carboskeletons,” Chem. Sci. 2015, 6, 2765–2769.
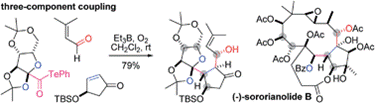
DOI: 10.1039/C5SC00457H
- S. Yoshioka, M. Nagatomo, M. Inoue, “Application of Two Direct C(sp3)-H Functionalizations for Total Synthesis of (+)-Lactacystin,” Org. Lett. 2015, 17, 90–93.
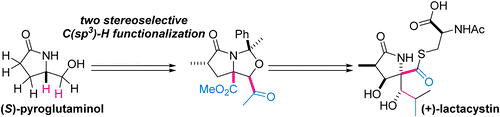
DOI: 10.1021/ol503291s
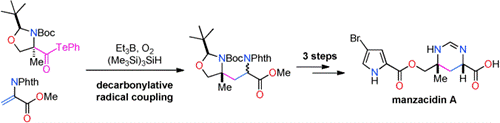
- M. Nagatomo, H. Nishiyama, H. Fujino, M. Inoue, “Decarbonylative Radical Coupling of α-Aminoacyl Tellurides: Single-Step Preparation of γ-Amino and α,β-Diamino Acids and Rapid Synthesis of Gabapentin and Manzacidin A,” Angew. Chem. Int. Ed. 2015, 54, 1537–1541.
(Selected as Hot Paper)
DOI: 10.1002/anie.201410186
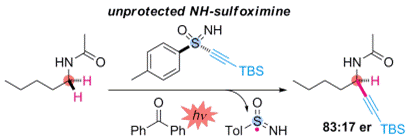
- M. Nagatomo, S. Yoshioka, M. Inoue, “Enantioselective Radical Alkynylation of C(sp3)-H Bonds Using Sulfoximine as a Traceless Chiral Auxiliary,” Chem. Asian. J. 2015, 10, 120–123.
DOI: 10.1002/asia.201402983
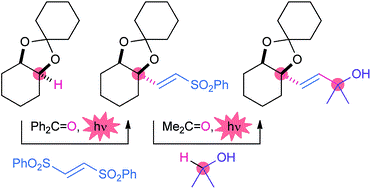
- Y. Amaoka, M. Nagatomo, M. Watanabe, K. Tao, S. Kamijo, M. Inoue, “Photochemically Induced Radical Alkenylation of C(sp3)-H Bonds,” Chem. Sci. 2014, 5, 4339–4345.
DOI: 10.1039/C4SC01631A
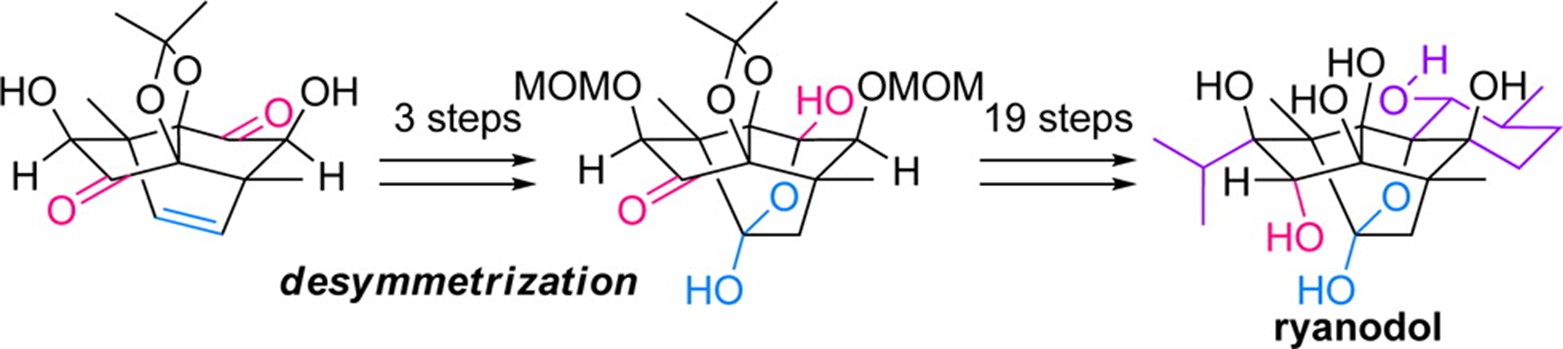
- M. Nagatomo, M. Koshimizu, K. Masuda, T. Tabuchi, D. Urabe, M. Inoue “Total Synthesis of Ryanodol,” J. Am. Chem. Soc. 2014, 136, 5916–5919.
DOI: 10.1021/ja502770n
- D. Kamimura, D. Urabe, M. Nagatomo, M. Inoue, “Et3B‐Mediated Radical-Polar Crossover Reaction for Single-Step Coupling of O,Te-Acetal, α,β-Unsaturated Ketones, and Aldehydes/Ketones,” Org. Lett. 2013, 15, 5122–5125.
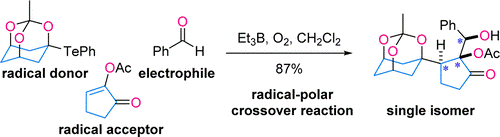
DOI: 10.1021/ol402563v
- Y. Amaoka, M. Nagatomo, M. Inoue, “Metal-Free Fluorination of C(sp3)-H Bonds Using a Catalytic N-Oxyl Radical,” Org. Lett. 2013, 15, 2160–2163.
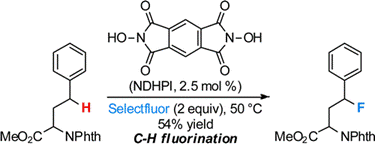
DOI: 10.1021/ol4006757
- D. Urabe, M. Nagatomo, K. Hagiwara, K. Masuda, M. Inoue, “Symmetry-Driven Synthesis of 9-Demethyl-10,15-dideoxyryanodol,” Chem. Sci. 2013, 4, 1615–1619.
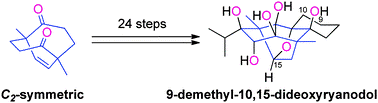
DOI: 10.1039/C3SC00023K
- M. Nagatomo, T. Nakata, “Stereoselective Synthesis of Maitotoxin GHI-Ring System Having a 1,2-Diol Side Chain,” Heterocycles 2008, 76, 1069–1074.
DOI: 10.3987/COM-08-S(N)113
Reviews and Accounts
- Y. Hikone, M. Nagatomo, M. Inoue, “Development of Streamlined Processes for Construction of Complex Natural Products: Three Synthesis of Resiniferatoxin,” J. Synth. Org. Chem., Jpn. 2023, 81, 1136–1149.
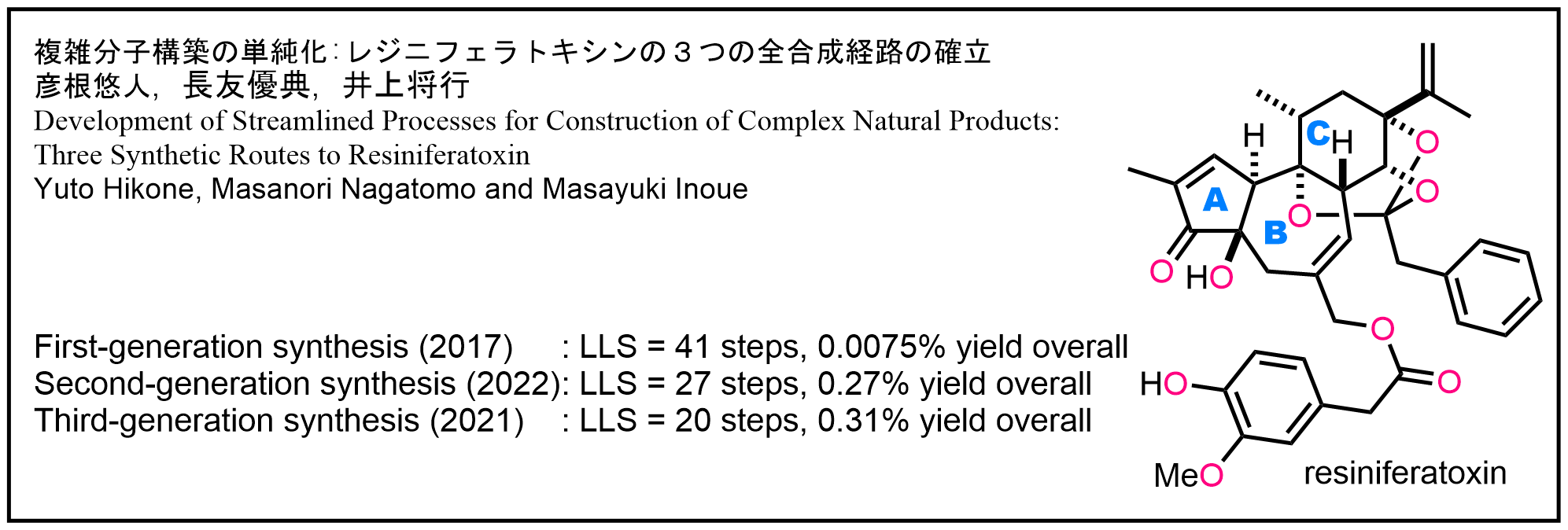
DOI: 10.5059/yukigoseikyokaishi.81.1136
- H. Fujino, M. Nagatomo, M. Inoue, “Total Syntheses of Hikosamine and Hikizimycin,” J. Org. Chem. 2021, 86, 16220–16230.
(Selected as the cover picture link)
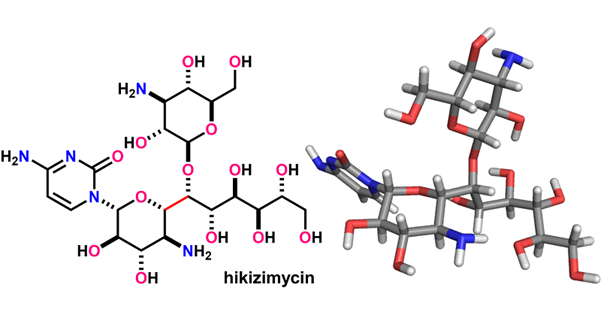
DOI: 10.1021/acs.joc.1c01773
- M. Nagatomo, M. Inoue, “Convergent Assembly of Highly Oxygenated Natural Products Enabled by Intermolecular Radical Reactions,” Acc. Chem. Res. 2021, 54, 595–604.
DOI: 10.1021/acs.accounts.0c00792
- M. Nagatomo, “Development of Synthetic Strategies for Densely Oxygenated Natural Products: Total Synthesis of Lactacystin and Zaragozic Acid C Using Photochemical C(sp3)–H Functionalization,” YAKUGAKU ZASSHI 2019, 139, 651–661.
DOI: 10.1248/yakushi.18-00210
- M. Nagatomo, “Discontent is the First Step in Progress-Learning from the Total Synthesis of Ryanodine,” J. Synth. Org. Chem., Jpn. 2018, 76, 494–497.
DOI: 10.5059/yukigoseikyokaishi.76.494
- M. Nagatomo, “Oxaziridine-Mediated Enantioselective Aminohydroxylation of Styrenes,” J. Synth. Org. Chem., Jpn. 2013, 71, 944–945.
DOI: 10.5059/yukigoseikyokaishi.71.944
Book Chapters
- 長友優典 ”環境が私を育み、私が環境を創る,” 有機合成化学協会誌 2025, 83, 666–669.
DOI: 10.5059/yukigoseikyokaishi.83.666
PDFへのリンクはこちら。
- 長友優典 “網羅的全合成戦略の探求 プリビレッジな構造の抽出と構築,” 化学と工業 飛翔する若手研究者, 日本化学会, pp 584–585 (2023).
- M. Nagatomo, “Total Syntheses of Densely Oxygenated Natural Products by Radical-Based Decarbonylative Convergent Assembly,” New Tide of Natural Product Chemistry, Springer, Singapore, pp 259–273 (2023).
DOI: 10.1007/978-981-99-1714-3_12
- Submitted by Y. Xue, A. Parsad, G. Dong, Checked by J. Han, M. Nagatomo, M. Inoue, “α-Arylation of Cyclopentanones by Palladium/Enamine Cooperative Catalysis,” Org. Synth. 2023, 100, 99–112.
DOI: 10.15227/orgsyn.100.0099
- Submitted by M. Varghese, H. E. Caputo, R. Xiao, A. Balijepalli, A. Hamoud, M. W. Grinstaff, Checked by K. Oga, M. Nagatomo, M. Inoue, “Stereoselective [2+2] Cycloadditions: Synthesis of a Tri-O-Bn-D-Glucal-derived β-Lactam,” Org. Synth. 2021, 98, 491–508.
DOI: 10.15227/orgsyn.098.0491
- Submitted by Y. Chen, Q. Chen, L. Tan, L. Chen, X. Wang, Checked by Y. Komori, M. Nagatomo, M. Inoue, “Preparation of 1H-Indazole-3-carbonitrile,” Org. Synth. 2020, 97, 314–326.
DOI: 10.15227/orgsyn.097.0314
- Submitted by J. Klepp, W. Dillon, Y. Lin, P. Feng, B. W. Greatrex, Checked by Y. Imamura, M. Nagatomo, M. Inoue, “Preparation of (-)-Levoglucosenone from Cellulose Using Sulfuric Acid in Polyethylene Glycol,” Org. Synth. 2020, 97, 38–53.
DOI: 10.15227/orgsyn.097.0038
- Submitted by Y. Kawashima, T. Furukawa, N. Chatani, M. Tobisu, Checked by T. Fukuda, M. Nagatomo, M. Inoue, “Nickel-Catalyzed Cross-Coupling of 2-Methoxynaphthalene with Methyl 4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)benzoate,” Org. Synth. 2019, 96, 36–52.
DOI: 10.15227/orgsyn.096.0036
- 長友優典, 井上将行, “リアノダンジテルペンの統一的全合成,” 天然有機化合物の全合成 独創的なものづくりの反応と戦略(CSJカレントレビュー 27) (日本化学会 編), 化学同人, pp 103–109 (2018).
Others
- 長友優典, 今村祐亮, 井上将行, “抗がん剤タキソールの全合成――ラジカル反応を活用した新しい合成戦略とその展開,” 化学 2023, 78 (7), 33–37.
(Associated Link: Link)
- 長友優典、”高酸化度天然物の全合成戦略の開発,” 薬事日報 2018, 11995, 21.
- 井上将行、 長友優典、占部大介、”ラジカル反応を基盤とした高酸化度天然物の収束的合成戦略,” ファルマシア 2017, 53(9), 860–864.
- 長友優典, 井上将行, “リアノジン類の統一的全合成-多様性を志向した共通中間体の設計戦略,” 化学 2016, 71(4), 47–48.
(Associated Link: Link)
- T. Hoshikawa, M. Nagatomo, M. Inoue, “Pentafluorophenyl Isocyanate,” e-EROS 2013, RN01621.
DOI: 10.1002/047084289X.rn01621















































