Overview
Our laboratory advances two overarching missions: “Visualization / Diagnosis” and “Controlled Drag Release / Therapeutics” Under “Visualization / Diagnosis,” we visualize specific biomolecules in vivo through molecular imaging to elucidate disease mechanisms and to enable early diagnosis and treatment. Under “Control,” we develop therapeutics that are activated only at target sites and establish methods for precise regulation and activation of compounds within living systems.
To Visualization / Diagnosis or Controlled Drag Release / Therapeutics life processes, we mainly harness nuclear medicine and light. In nuclear medicine, we handle radionuclides such as 18F for positron emission tomography (PET) and alpha-emitters like 211At, as well as Auger electron–emitting nuclides including 191Pt. In optics, beyond the widely used visible–near‑infrared fluorescence, we also explore X‑rays (shorter wavelengths) and photoacoustics that leverage heat released from photoexcited molecules.
By integrating nuclear medicine and light to see and control biology, we aim to clarify pathologies and create new modalities for diagnosis and therapy.
Visualization / Diagnosis
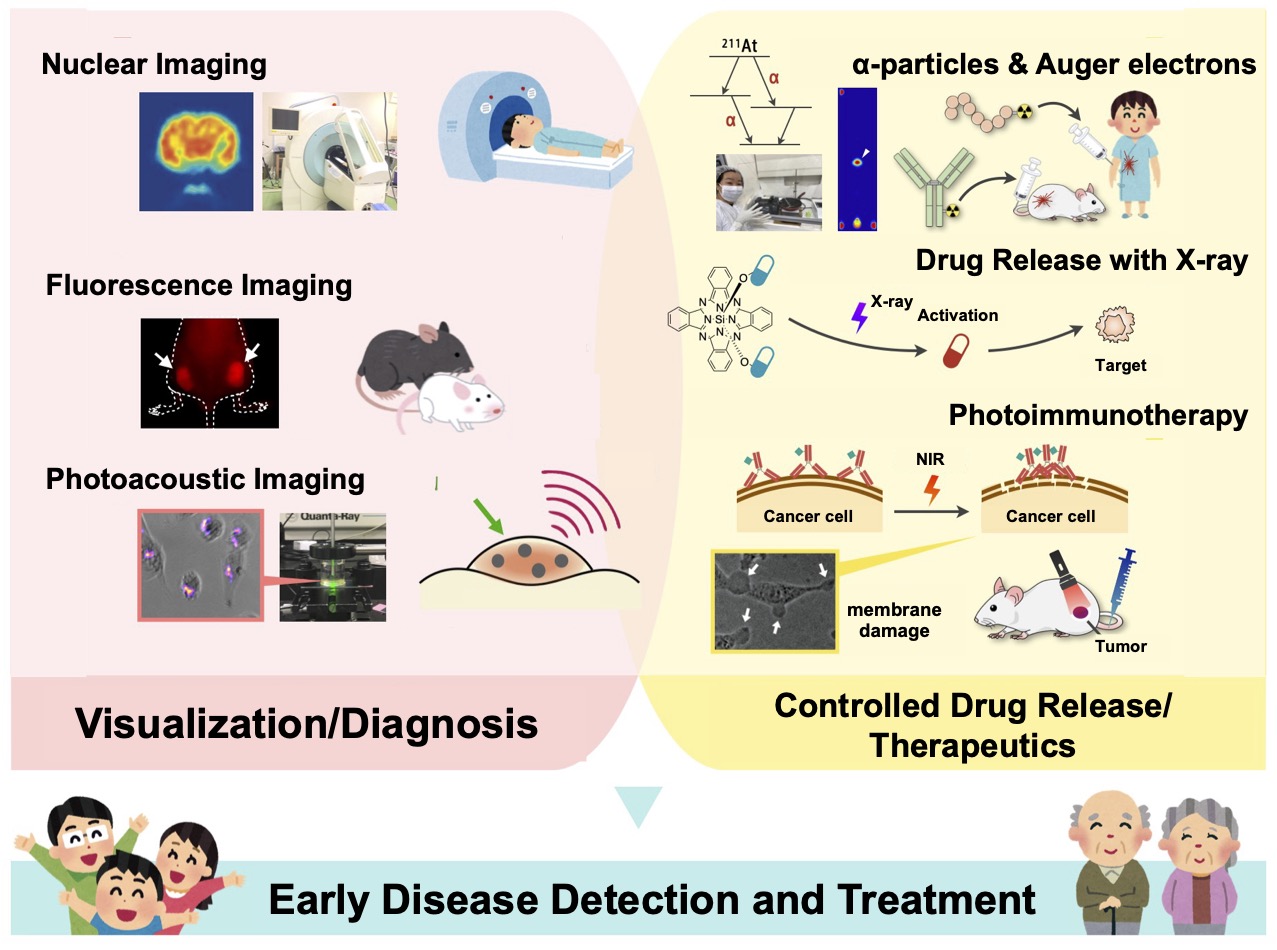
Nuclear Medicine Imaging
Nuclear medicine imaging visualizes specific molecules in vivo using positron-emitting nuclides such as 18F. For example, tumors often metabolize more glucose than normal tissues; thus, the PET tracer [18F]FDG (glucose labeled with 18F) is widely used clinically. Nuclear medicine provides high quantitativity and whole‑body coverage, making it valuable for early detection and therapy assessment.
We analyze in vivo changes induced by treatments in cancer and inflammatory disease models via nuclear medicine imaging, and we work to establish robust imaging‑based methods for evaluating therapeutic efficacy.
Selected Publications
- . PD1 blockade alters cell-cycle distribution and affects 3'-deoxy-3'-[18F]fluorothymidine uptake in a mouse CT26 tumor model. Ann Nucl Med 2022. Online ahead of print. PMID 35969311
- . Reduction of tumor hypoxia by anti-PD-1 therapy assessed using pimonidazole and [18F]FMISO. Nucl Med Biol 2022, 108–109: 85–92. PMID 35367730
- . Uptake of nicotinic acetylcholine receptor imaging agent is reduced in the pro-inflammatory macrophage. Nucl Med Biol 2021, 102–103: 45–55. PMID 34619460
- . Influence on [18F]FDG uptake by cancer cells after anti PD-1 therapy in an enforced-immune activated mouse tumor. EJNMMI Res 2020, 10(1): 24. PMID 32189078
- . Anti PD-1 treatment increases [18F]FDG uptake by cancer cells in a mouse B16F10 melanoma model. EJNMMI Research 2018, 8: 82. PMID 30117062
Related Grants
- KAKENHI (Grant-in-Aid for Scientific Research B): “Evaluation of tumor microenvironment by nuclear medicine imaging focused on energy metabolism” (PI: Mikako Ogawa)
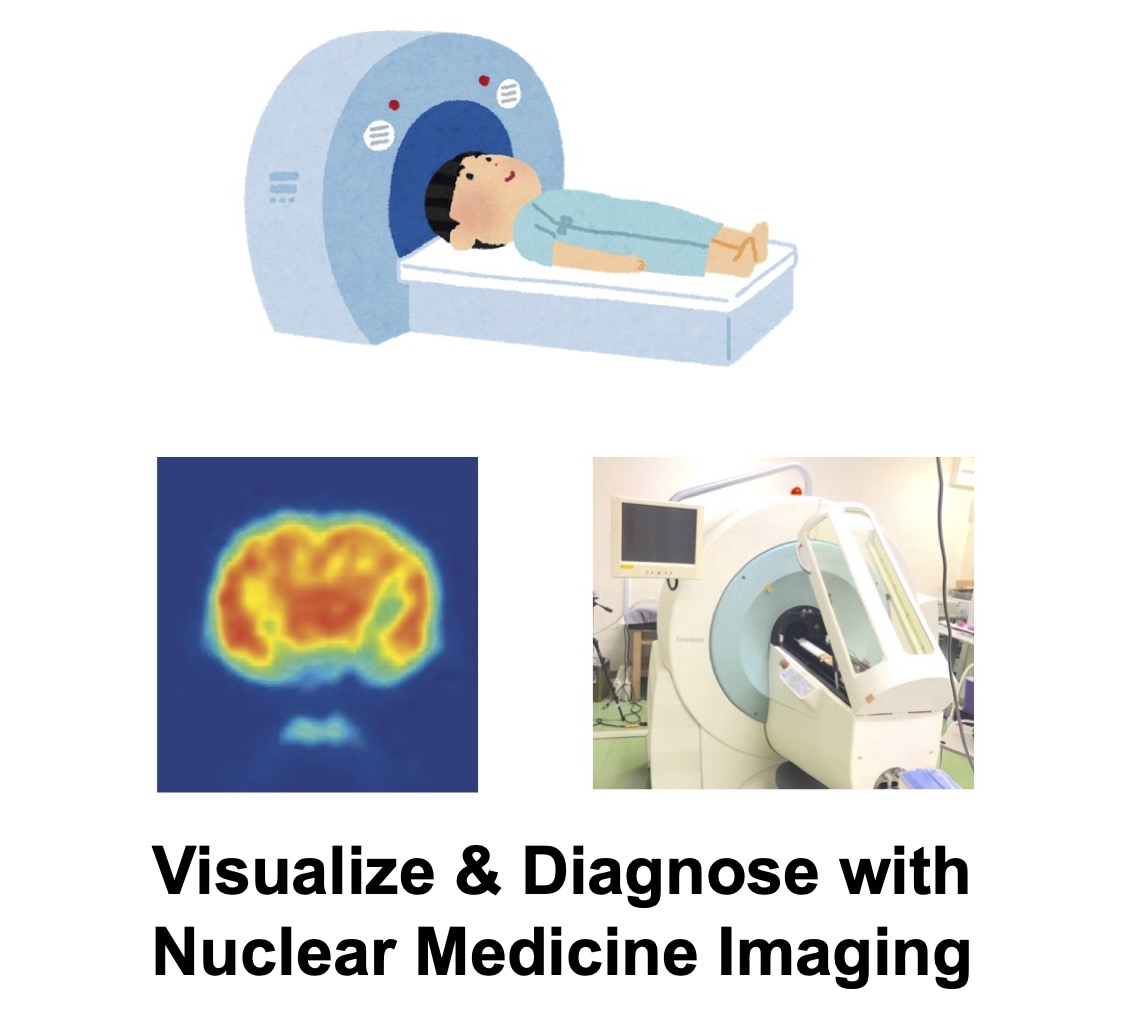
Fluorescence & Photoacoustic Imaging
Fluorescence imaging uses molecules that emit fluorescence after light absorption to track drug biodistribution and probe biological functions. We also advance photoacoustic imaging, which detects acoustic waves generated from photoexcited molecules. Compared with light, acoustic waves are less attenuated by tissue, enabling imaging at greater depths.
We integrate fluorescence imaging for pharmacokinetic analyses and develop new fluorescent probes for diagnosis. In photoacoustics, we design agents whose signals change only at target sites, achieving high‑contrast lesion visualization.
Selected Publications
- . Correction: Takakura et al. In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties. Cancers 2021, 13, 2245. Cancers (Basel) 2022, 14(8): 1880. PMID 35454954 (Correction to PMID 34067065)
Controlled Drag Release / Therapeutics
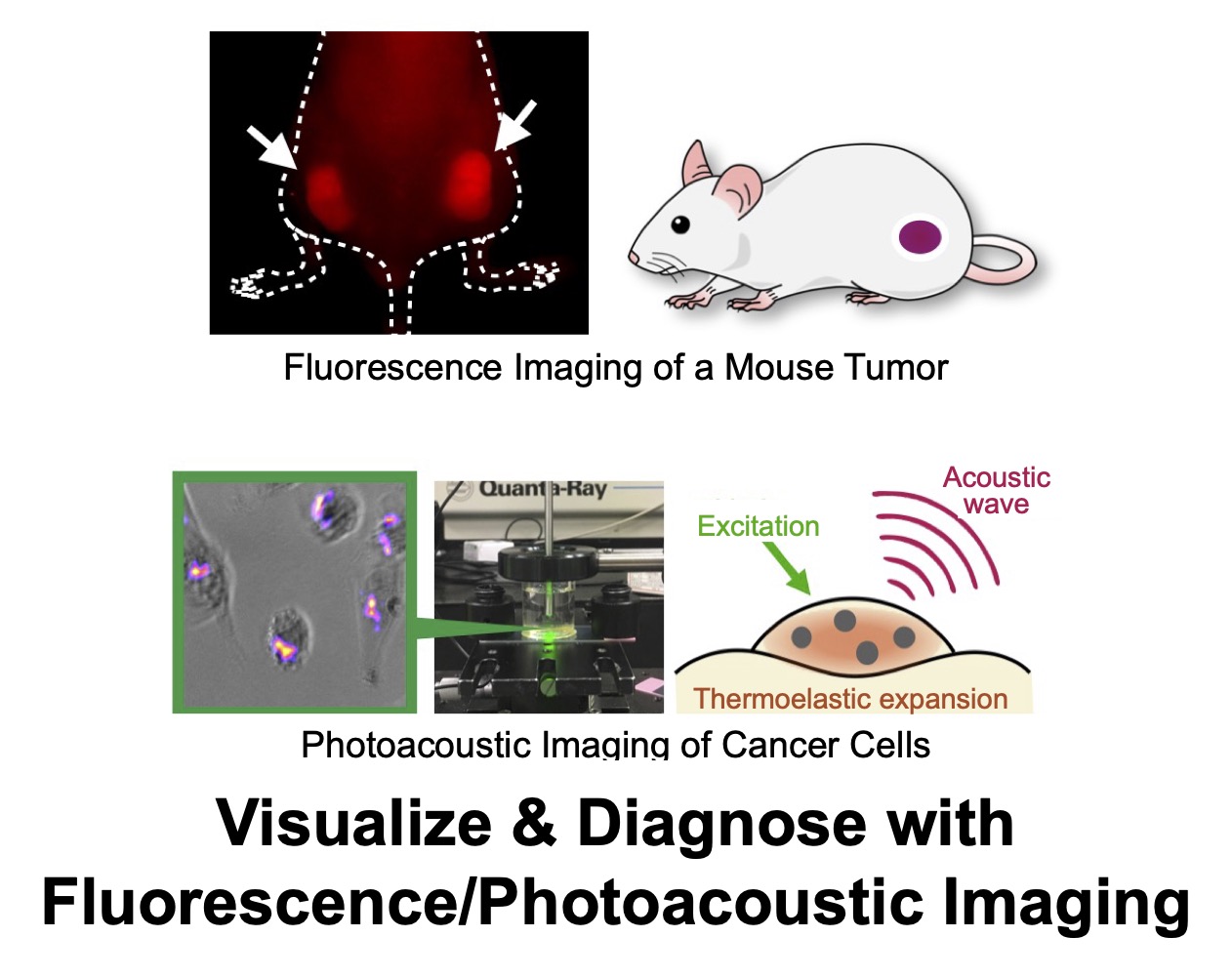
Alpha Particles & Auger Electrons
Beta‑emitting radioisotopes have long been used in cancer therapy. Recently, however, alpha particles and Auger electrons—with much shorter ranges than beta particles—have drawn attention. In tissue, alphas travel only a few cells and Auger electrons only a few molecules, confining dose to extremely narrow regions and enabling highly selective ablation of target molecules and cells.
We establish stable labeling methods for alpha‑ and Auger‑emitting nuclides and evaluate therapeutic effects of the labeled agents. In particular, we investigate 211At (alpha emitter) and 191Pt (Auger emitter) to define clinical opportunities for each modality.
Selected Publications
- . Development of novel 191Pt-labeled Hoechst33258: 191Pt is more suitable than 111In for targeting DNA. J Med Chem 2022, 65(7): 5690–5700. PMID 35358392
- . Transition-metal-free nucleophilic 211At-astatination of spirocyclic aryliodonium ylides. Org Biomol Chem 2021, 19(25): 5525–5528. PMID 34124736
- . In Vitro Evaluation of No-carrier-added Radiolabeled Cisplatin ([189, 191Pt]cisplatin) Emitting Auger Electrons. Int J Mol Sci 2021, 22: 4622. PMID 33924843
- . Synthesis of no-carrier-added [188, 189, 191Pt]cisplatin from a cyclotron produced 188, 189, 191PtCl42-) complex. Sci Rep 2021, 11: 8140. PMID 33854142
Related Grants
- AMED: “Development of an automated solid‑phase synthesizer for astatine‑labeled compounds” (PI: Mikako Ogawa)
X‑ray‑Triggered Activation of Compounds
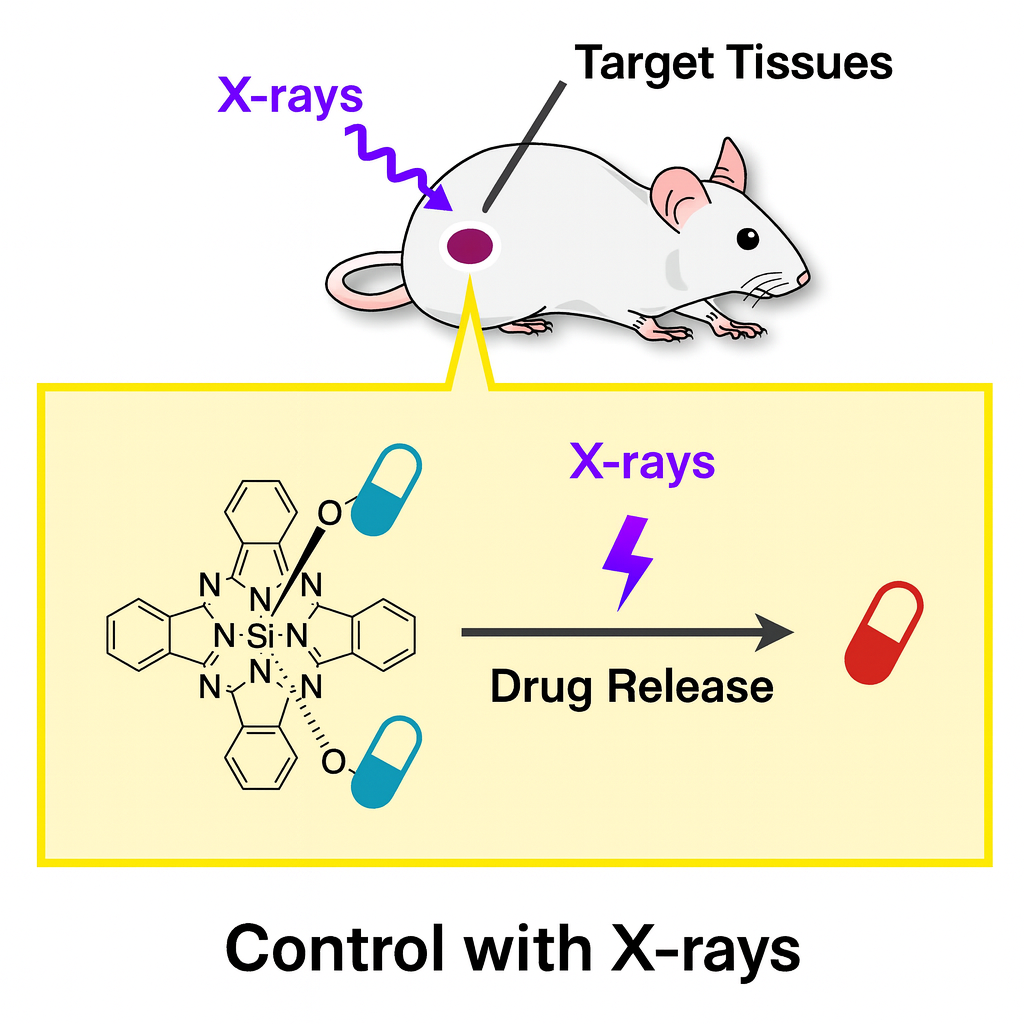
Caged compounds release bioactive molecules upon external stimuli (e.g., light), enabling localized control of biological functions. To date, activation typically relies on UV–visible light due to energy requirements, but limited tissue penetration constrains in vivo use to superficial regions (e.g., mouse brain cortex), hindering translation to humans.
We therefore focus on X‑rays, which penetrate deep tissues, as an external trigger for caged compounds in vivo. We search for structures that undergo deprotection upon X‑ray irradiation and develop agents activated only within the irradiated area, aiming to treat lesions while minimizing side effects on healthy tissues.
Selected Publications
- . Ligand release from silicon phthalocyanine dyes triggered by X‑ray irradiation. Org Biomol Chem 2022. Online ahead of print. PMID 35972402
- . Axial‑ligand‑cleavable silicon phthalocyanines triggered by near‑infrared light toward design of photosensitizers for photoimmunotherapy. J Photochem Photobiol A: Chem 2022, 426(1): 113749. DOI 10.1016/j.jphotochem.2021.113749
Related Grants
- JST CREST [Optical Innovations]: “Light‑based molecular control in deep human tissues” (PI: Mikako Ogawa)
Near‑Infrared Photoimmunotherapy (PIT)
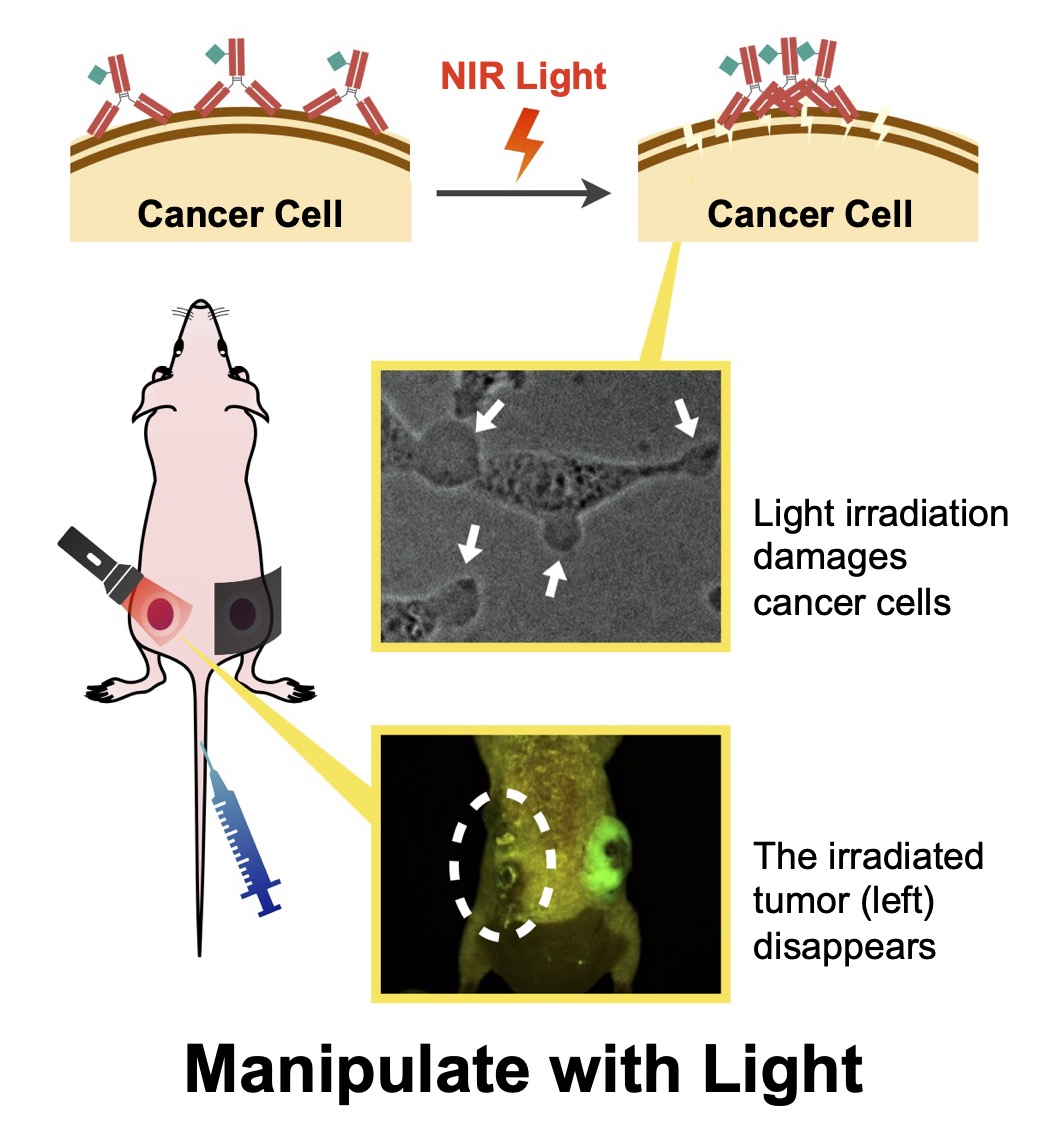
Near‑infrared photoimmunotherapy (PIT) is a cancer therapy that selectively kills tumor cells by irradiating NIR‑responsive agents localized at tumors. PIT spares surrounding normal cells and stimulates host immunity, yielding strong therapeutic effects. However, incomplete understanding of the cytotoxic mechanism hinders broader drug discovery.
We dissect PIT mechanisms using multiple approaches. For example, we discovered that photoresponsive agents aggregate upon light exposure and physically disrupt cancer cell membranes. We also develop novel agents that react efficiently even to weak light, aiming for higher therapeutic efficacy.
Selected Publications
- . EPR Characterisation of Phthalocyanine Radical Anions in Near‑Infrared Photocleavage of the Hydrophilic Axial Ligand of a Photoimmunotherapeutic Reagent, IR700. ChemPhotoChem 2022, 6(1): e202100172. DOI 10.1002/cptc.202100172 (Cover Profile: DOI 10.1002/cptc.202100277)
- . Comparison of low‑molecular‑weight ligand and whole antibody in prostate‑specific membrane antigen targeted near‑infrared photoimmunotherapy. Int J Pharm 2021, 609: 121135. *Contributed equally. PMID 34571072
- . Analysis of the triplet‑state kinetics of a photosensitizer for photoimmunotherapy by fluorescence correlation spectroscopy. J Photochem Photobiol A: Chem 2021, 408: 113094.
- . Theoretical and Experimental Studies on the Near‑Infrared Photoreaction Mechanism of a Silicon Phthalocyanine Photoimmunotherapy Dye: Photoinduced Hydrolysis by Radical Anion Generation. ChemPlusChem 2020, 85: 1–6. PMID 32449613 (Cover Profile: PMID 32830450)
- . Changes in plasma membrane damage inducing cell death after treatment with near‑infrared photoimmunotherapy. Cancer Sci 2018, 109(9): 2889–2896. PMID 29949672
Related Grants
- JST PRESTO [Optical Extremes]: “Developing cancer therapies using novel light–biological interactions” (PI: Mikako Ogawa)