研究業績
原著論文
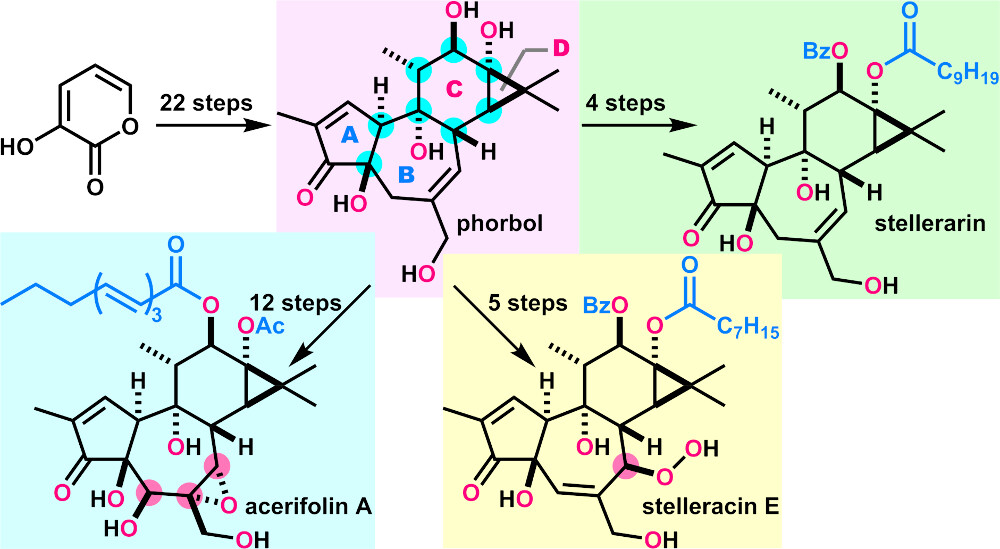
- A. Watanabe, M. Nagatomo, A. Hirose, Y. Hikone, N. Kishimoto, S. Miura, T. Yasutake, T. Abe, S. Misumi, M. Inoue, “Total Syntheses of Phorbol and 11 Tigliane Diterpenoids and Their Evaluation as HIV Latency-Reversing Agents,” J. Am. Chem. Soc. 2024, 146, 8746–8756.
(UTokyo FOCUS内 press releasesにて紹介されました。リンク)
(24/3/18 日本経済新聞にて報道されました。リンク)
DOI: 10.1021/jacs.4c01589
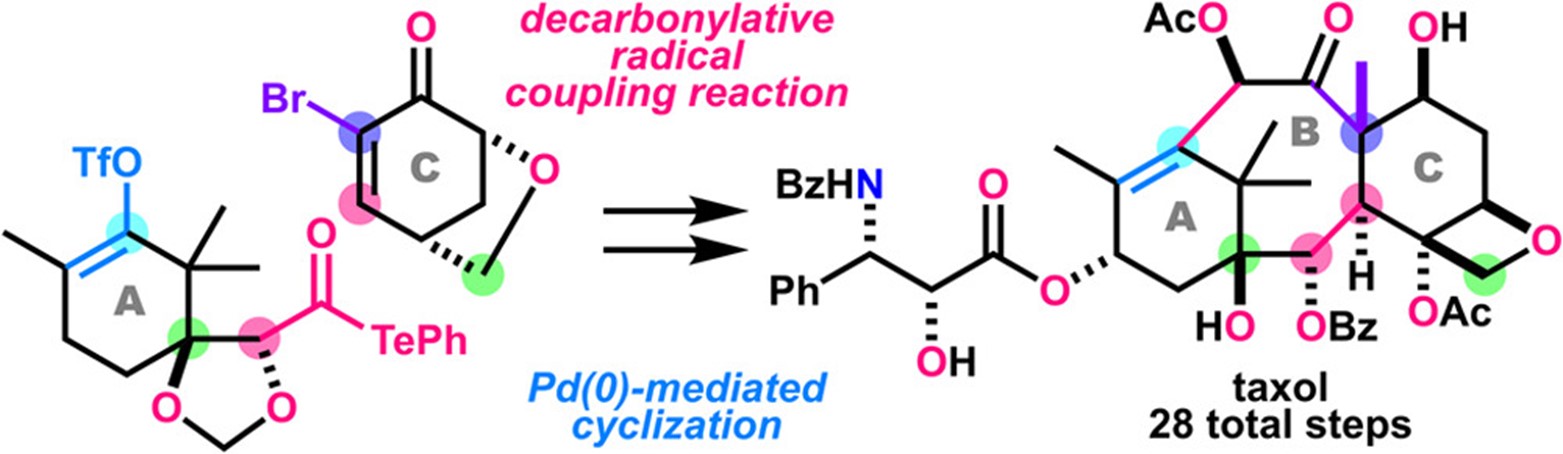
- T. Watanabe, K. Oga, H. Matoba, M. Nagatomo, M. Inoue, “Total Synthesis of Taxol Enabled by Intermolecular Radical Coupling and Pd-Catalyzed Cyclization,” J. Am. Chem. Soc. 2023, 145, 25894–25902.
(UTokyo FOCUS内 press releasesにて紹介されました。リンク)
(23/11/21 日本経済新聞にて報道されました。リンク)
(23/12/4 Selected as Most Read Articles)
(24/1/9 Featured in “Some Items of Interest to Process R&D Chemists and Engineers” Org. Process. Res. Dev. 2024, 28, 1.)
(Featured in Synfacts)
(Chem-Stationにて取り上げられました。)
DOI: 10.1021/jacs.3c10658
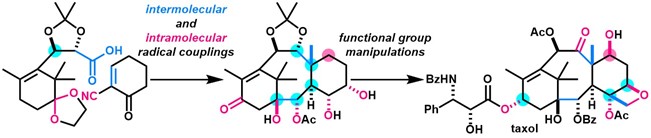
- Y. Imamura, K. Takaoka, Y. Komori, M. Nagatomo, M. Inoue, “Total Synthesis of Taxol Enabled by Inter- and Intramolecular Radical Coupling Reactions,” Angew. Chem. Int. Ed. 2023, 62, e202219114.
(UTokyo FOCUS内 press releasesにて紹介されました。リンク)
(23/1/23 日本経済新聞にて報道されました。リンク)
(Selected as Hot Paper)
(Chem-Stationにて取り上げられました。)
(Featured in Synfacts)
DOI: 10.1002/anie.202219114
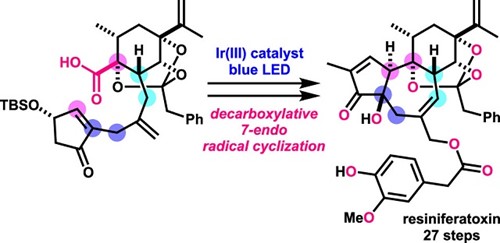
- Y. Hikone, T. Kato, M. Nagatomo, M. Inoue, “Total Synthesis of Resiniferatoxin Enabled by Photocatalytic Decarboxylative Radical Cyclization,” Org. Lett. 2022, 24, 929–933.
(Selected as Most Read Articles)
DOI: 10.1021/acs.orglett.1c04286
- D. Kuwana, Y. Komori, M. Nagatomo, M. Inoue, “Photoinduced Decarboxylative Radical Coupling Reaction of Multiply Oxygenated Structures by Catalysis of Pt-Doped TiO2,” J. Org. Chem. 2022, 87, 730–736.
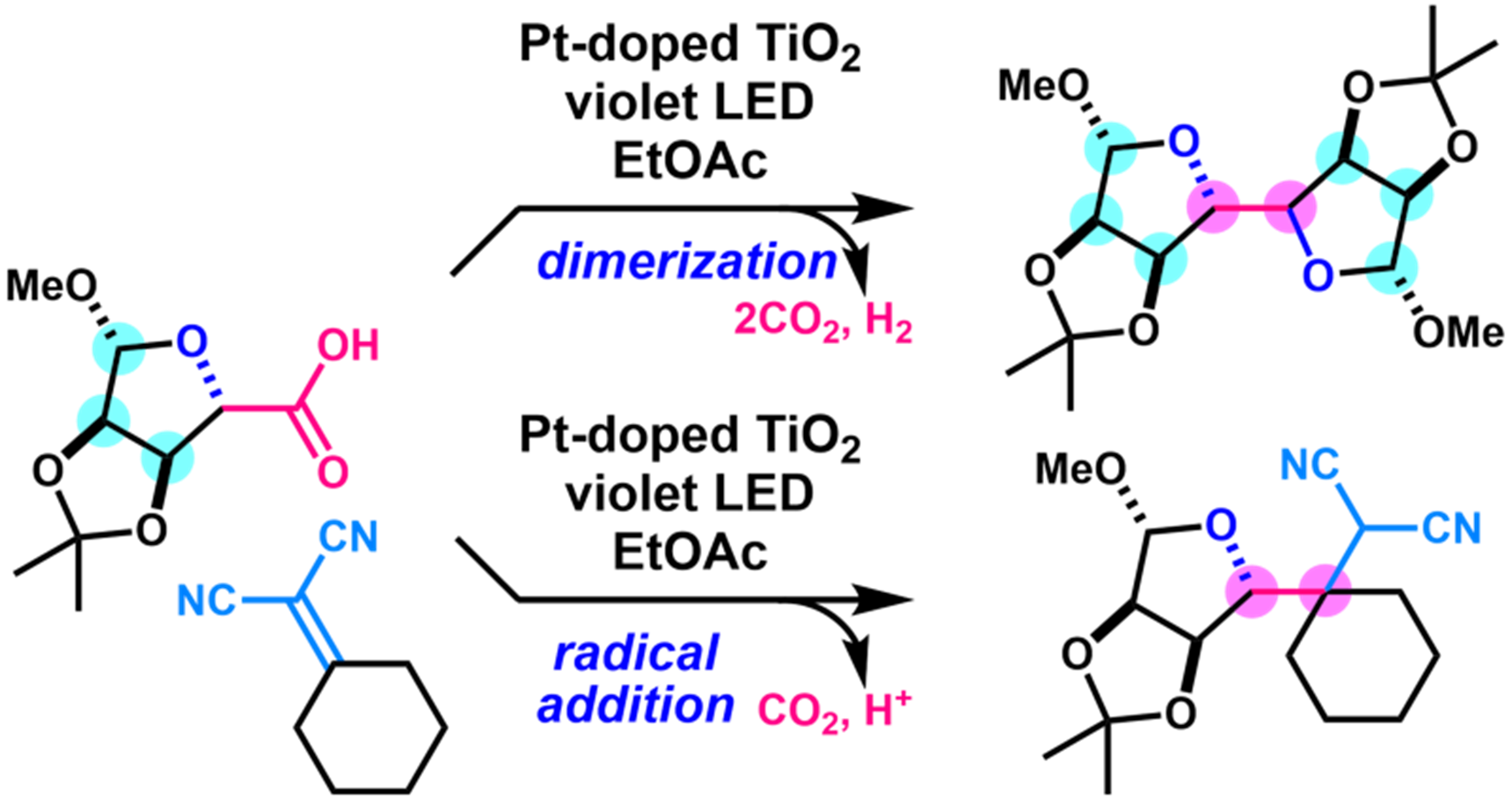
DOI: 10.1021/acs.joc.1c02736
- A. Hirose, A. Watanabe, K. Ogino, M. Nagatomo, M. Inoue, “Unified Total Syntheses of Rhamnofolane, Tigliane, and Daphnane Diterpenoids,” J. Am. Chem. Soc. 2021, 143, 12387–12396.
(Featured in Synfacts)
(Organic Chemistry Highlightsで紹介されました。リンク)
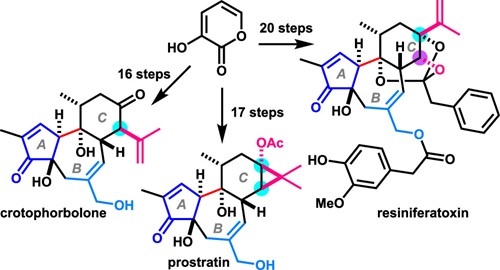
DOI: 10.1021/jacs.1c06450
- M. Nagatomo, K. Zhang, H. Fujino, M. Inoue, “Et3B/Et2AlCl/O2‐Mediated Radical Coupling Reaction between α‐Alkoxyacyl Tellurides and 2‐Hydroxybenzaldehyde Derivatives,” Chem. Asian. J. 2020, 15, 3820–3824.
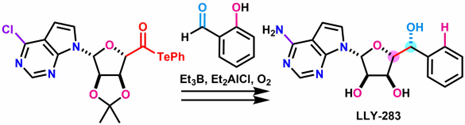
DOI: 10.1002/asia.202001090
- T. Fukuda, M. Nagatomo, M. Inoue, “Total Synthesis of Diospyrodin and Its Three Diastereomers,” Org. Lett. 2020, 22, 6468–6472.
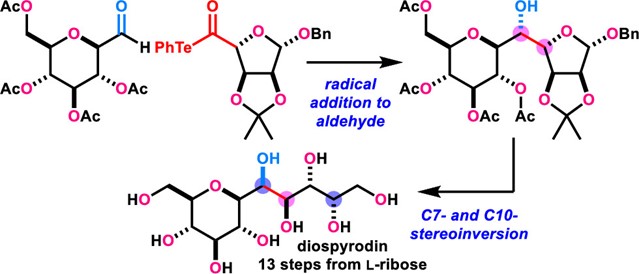
DOI: 10.1021/acs.orglett.0c02280
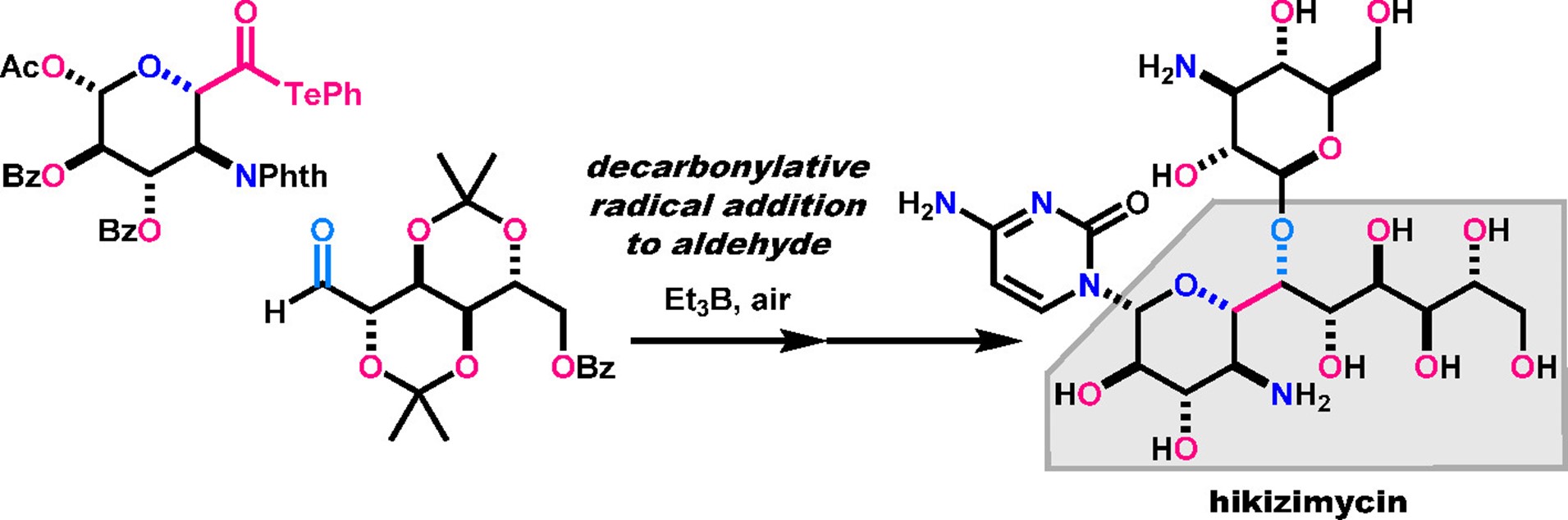
- H. Fujino, T. Fukuda, M. Nagatomo, M. Inoue, “Convergent Total Synthesis of Hikizimycin Enabled by Intermolecular Radical Addition to Aldehyde,” J. Am. Chem. Soc. 2020, 142, 13227–13234.
(UTokyo FOCUS内 articlesにて紹介されました。 リンク)
(Organic Chemistry Highlightsで紹介されました。リンク)
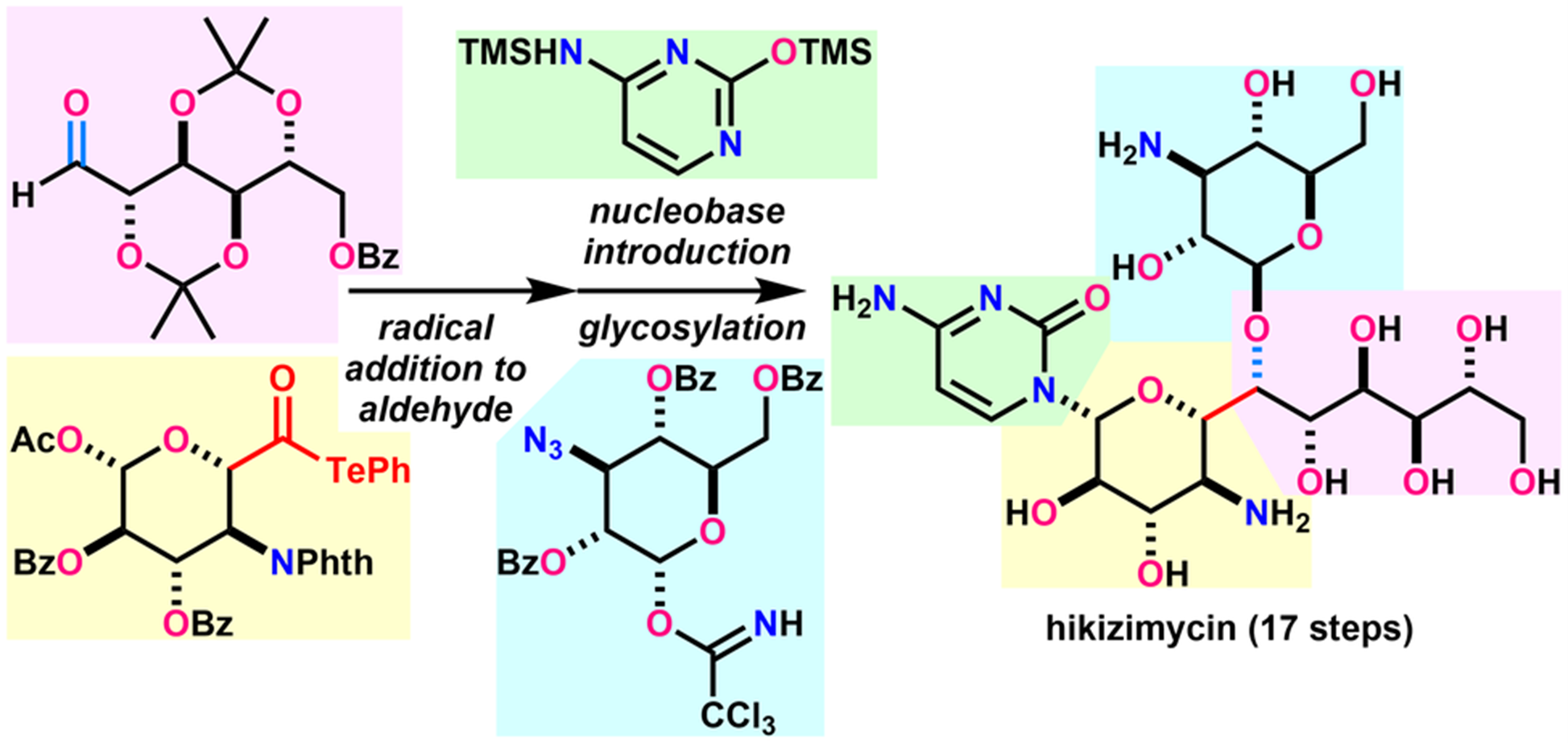
DOI: 10.1021/jacs.0c06354
- D. Kuwana, M. Nagatomo, M. Inoue, “Total Synthesis of 5-epi-Eudesm-4(15)-ene-1β,6β-diol via Decarbonylative Radical Coupling Reaction,” Org. Lett. 2019, 21, 7619–7623.
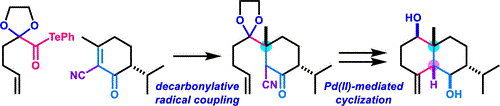
DOI: 10.1021/acs.orglett.9b02895
- Y. Imamura, S. Yoshioka, M. Nagatomo, M. Inoue, “Total Synthesis of 1‐Hydroxytaxinine,” Angew. Chem. Int. Ed. 2019, 58, 12159–12163.
(Selected as Hot Paper)
(UTokyo FOCUS内 articlesにて紹介されました。 リンク)
(Chem-Stationで紹介されました。リンク)
(Organic Chemistry Highlightsで紹介されました。リンク)
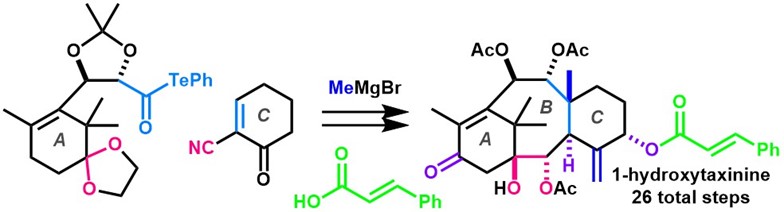
DOI: 10.1002/anie.201906872
- D. Kuwana, B. Ovadia, D. Kamimura, M. Nagatomo, M. Inoue, “Installation of O‐Heterocycles to N‐Heteroarenes via an Et3B/O2‐mediated Radical Reaction of α-Alkoxy and α-Alkoxyacyl Tellurides,” Asian J. Org. Chem. 2019, 8, 1088–1091.
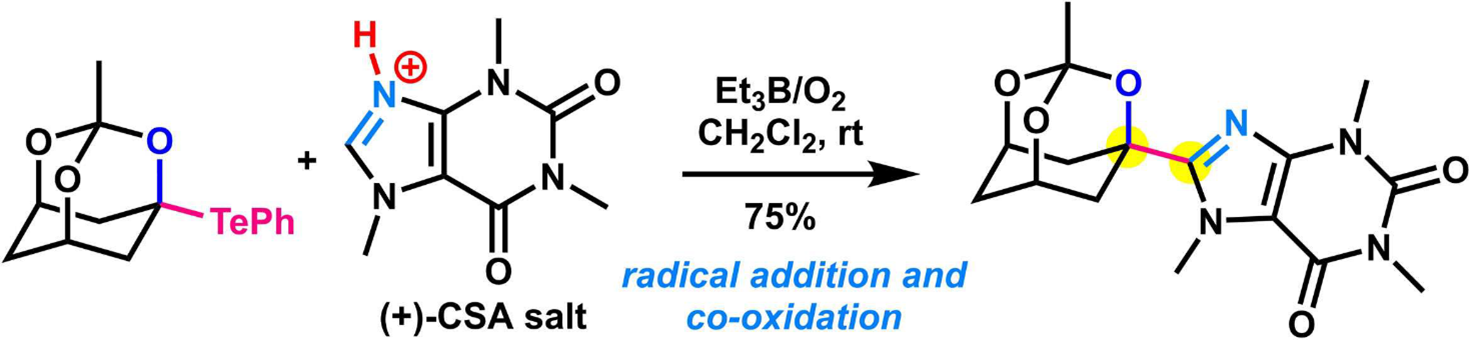
DOI: 10.1002/ajoc.201900170
- M. Nagatomo, Y. Fujimoto, K. Masuda, M. Inoue, “Construction of a 6/5/9-membered tricyclic structure of cladiellins via radical-polar crossover reaction,” J. Antibiot. 2019, 72, 486–489.
DOI: 10.1038/s41429-019-0150-7
- H. Matoba, T. Watanabe, M. Nagatomo, M. Inoue, “Convergent Synthesis of Taxol Skeleton via Decarbonylative Radical Coupling Reaction,” Org. Lett. 2018, 20, 7554–7557.
(Selected as Most Read Articles of the month)
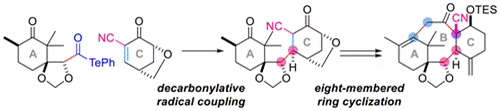
DOI: 10.1021/acs.orglett.8b03302
- T. Kawamata, A. Yamaguchi, M. Nagatomo, M. Inoue, “Convergent Total Synthesis of Asimicin via Decarbonylative Radical Dimerization,” Chem. Eur. J. 2018, 24, 18907–18912.
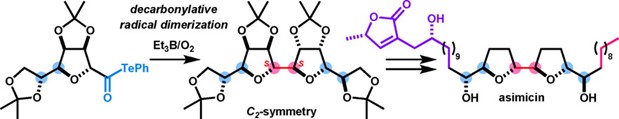
DOI: 10.1002/chem.201805317
- D. Urabe, Y. Nakagawa, K. Mukai, K. Fukushima, N. Aoki, H. Itoh, M. Nagatomo, M. Inoue, “Total synthesis and biological evaluation of 19-hydroxysarmentogenin-3-O-a-L-rhamnoside, trewianin, and their aglycons,” J. Org. Chem. 2018, 83, 13888–13910.
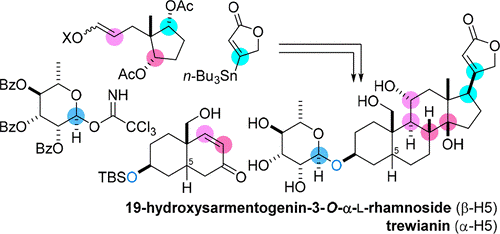
DOI: 10.1021/acs.joc.8b02219
- M. Koshimizu, M. Nagatomo, M. Inoue, “Construction of a pentacyclic ring system of isoryanodane diterpenoids by SmI2-mediated transannular cyclization,” Tetrahedron. 2018, 74, 3384–3390.
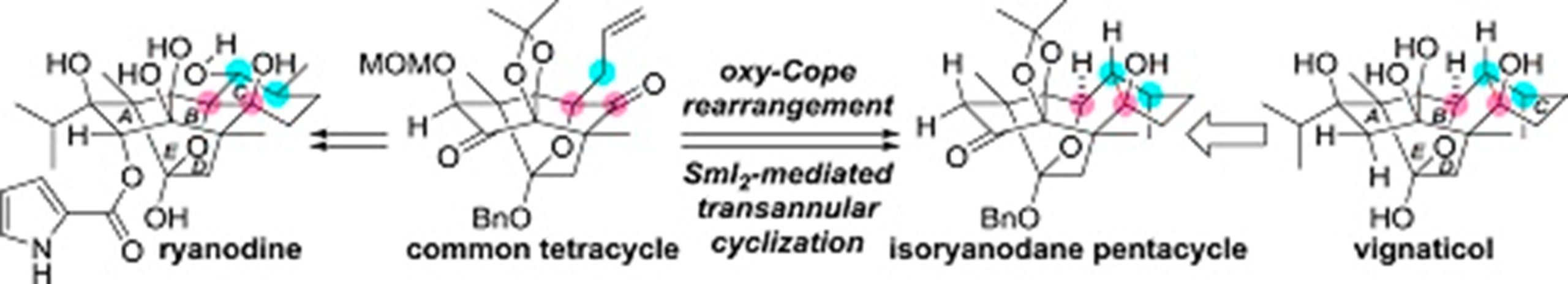
DOI: 10.1016/j.tet.2018.03.061
- H. Fujino, M. Nagatomo, A. Paudel, S. Panthee, H. Hamamoto, K. Sekimizu, M. Inoue, “Unified Total Synthesis of Polyoxin J, L, and Their Fluorinated Analogues on the Basis of Decarbonylative Radical Coupling Reactions,” Angew. Chem. Int. Ed. 2017, 56, 11865–11869.
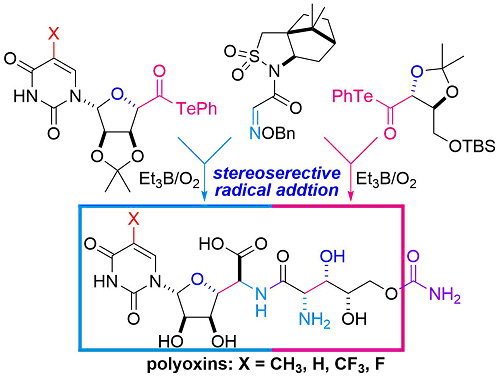
DOI: 10.1002/anie.201706671
- K. Masuda, M. Tanigawa, M. Nagatomo, D. Urabe, M. Inoue, “Construction of carbocycles initiated by Cu-catalyzed radical reaction of Cl2C(CN)2,” Tetrahedron 2017, 73, 3596–3605.
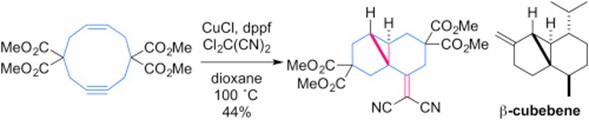
DOI: 10.1016/j.tet.2017.03.063
- T. Kawamata, M. Nagatomo, M. Inoue, “Total Synthesis of Zaragozic Acid C: Implementation of Photochemical C(sp3)-H Acylation,” J. Am. Chem. Soc. 2017, 139, 1814–1817.
(Highlighted in Synfacts 2017, 13, 0337. and selected as its Cover Picture)
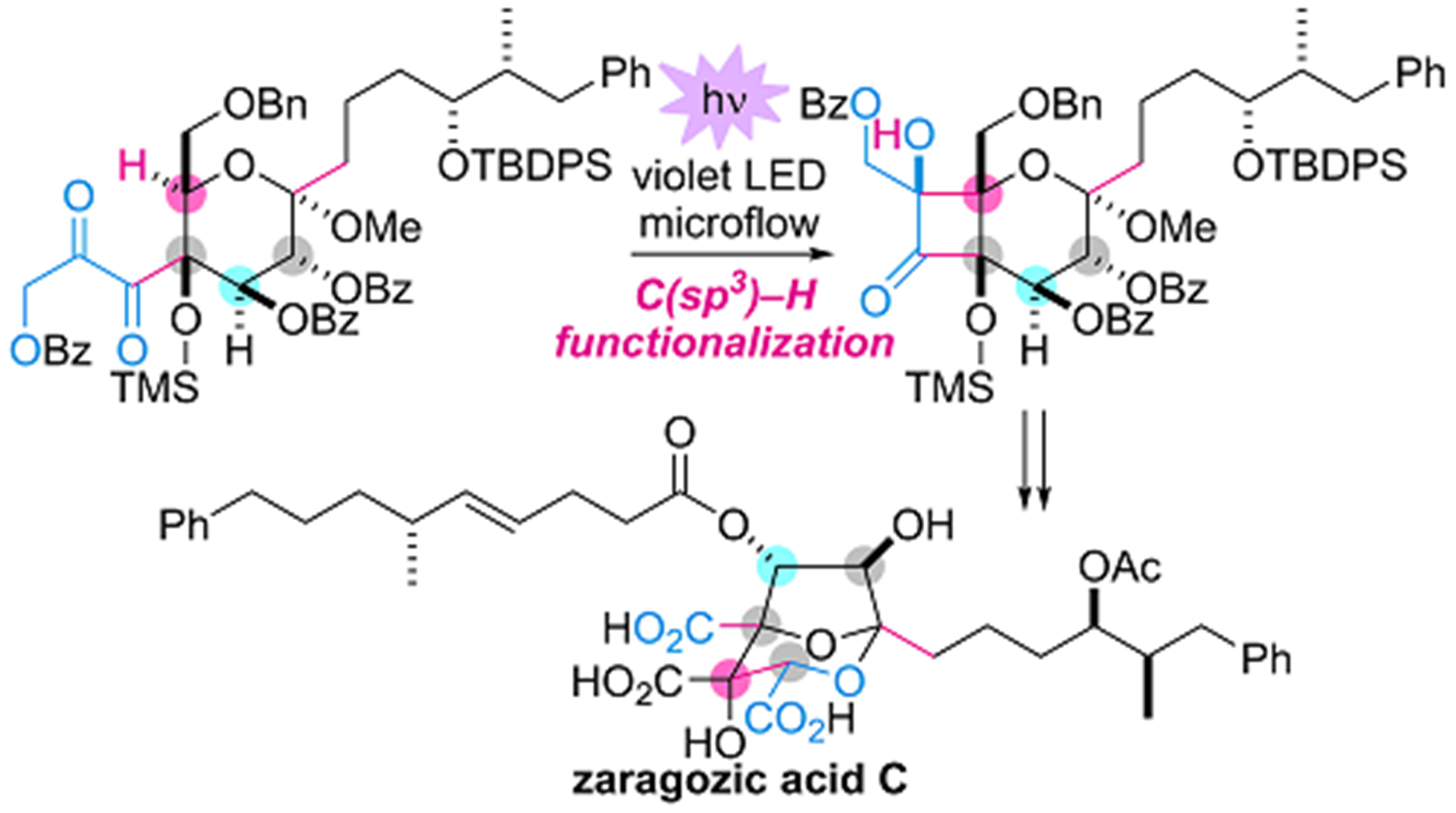
DOI: 10.1021/jacs.6b13263
- K. Masuda, M. Nagatomo, M. Inoue, “Direct assembly of multiply oxygenated carbon chains by decarbonylative radical-radical coupling reactions,” Nature Chem. 2017, 9, 207–212.
(Highlighted in Synfacts 2017, 13, 0534. )
(Associated Link: News and Views – “Organic chemistry: A radical step forward”)
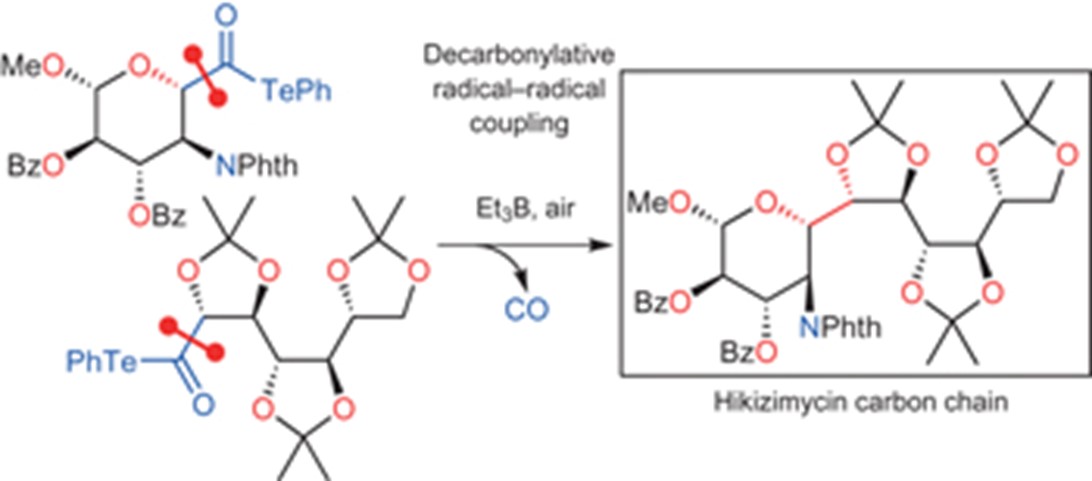
DOI: 10.1038/nchem.2639
- S. Matsumura, Y. Matsui, M. Nagatomo, M. Inoue, “Stereoselective construction of anti– and syn-1,2-diol structures via decarbonylative radical coupling of α-alkoxyacyl tellurides,” Tetrahedron 2016, 72, 4859–4866.
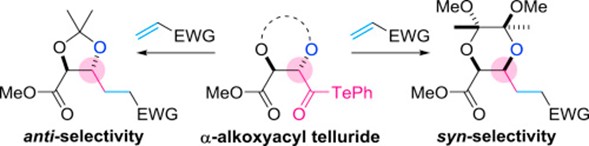
DOI: 10.1016/j.tet.2016.06.056
- D. Kamimura, M. Nagatomo, D. Urabe, M. Inoue, “Expanding the scope of Et3B/O2-mediated coupling reactions of O,Te-acetal,” Tetrahedron 2016, 72, 7839–7848.
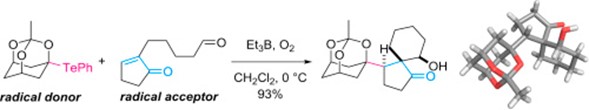
DOI: 10.1016/j.tet.2016.04.023
- K. Masuda, M. Nagatomo, M. Inoue, “Chemical Conversion of Ryanodol to Ryanodine,” Chem. Pharm. Bull. 2016, 64, 874–879.
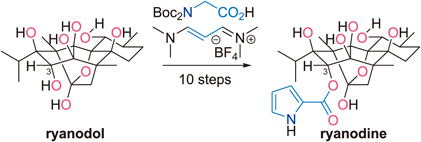
DOI: 10.1248/cpb.c16-00214
- M. Koshimizu, M. Nagatomo, M. Inoue, “Unified Total Synthesis of 3-epi-Ryanodol, Cinnzeylanol, Cinncassiols A and B, and Structural Revision of Natural Ryanodol and Cinnacasol,” Angew. Chem. Int. Ed. 2016, 55, 2493–2497.
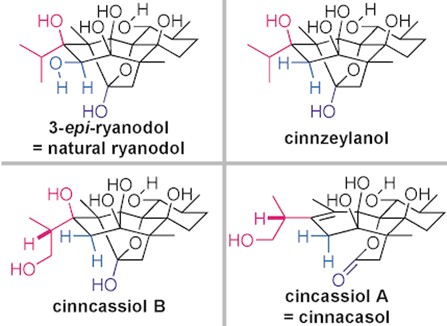
DOI: 10.1002/anie.201511116
- K. Masuda, M. Koshimizu, M. Nagatomo, M. Inoue, “Asymmetric Total Synthesis of (+)-Ryanodol and (+)-Ryanodine,” Chem. Eur. J. 2016, 22, 230–236.
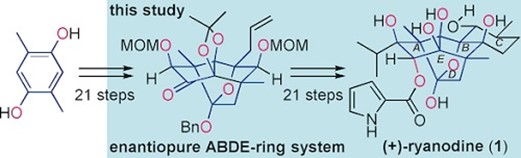
DOI: 10.1002/chem.201503641
- M. Nagatomo, K. Hagiwara, K. Masuda, M. Koshimizu, T. Kawamata, Y. Matsui, D. Urabe, M. Inoue, ” Symmetry-Driven Strategy for the Assembly of the Core Tetracycle of (+)-Ryanodine: Synthetic Utility of a Cobalt-Catalyzed Olefin Oxidation and α-Alkoxy Bridgehead Radical Reaction,” Chem. Eur. J. 2016, 22, 222–229.
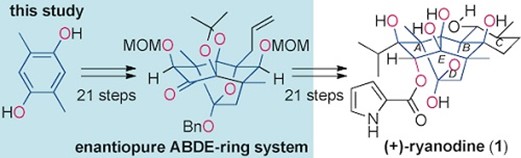
DOI: 10.1002/chem.201503640
- M. Nagatomo, D. Kamimura, Y. Matsui, K. Masuda, M. Inoue, “Et3B-Mediated Two- and Three-Component Coupling Reactions via Radical Decarbonylation of α-Alkoxyacyl Tellurides: Single-Step Construction of Densely Oxygenated Carboskeletons,” Chem. Sci. 2015, 6, 2765–2769.
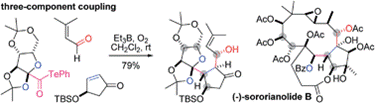
DOI: 10.1039/C5SC00457H
- S. Yoshioka, M. Nagatomo, M. Inoue, “Application of Two Direct C(sp3)-H Functionalizations for Total Synthesis of (+)-Lactacystin,” Org. Lett. 2015, 17, 90–93.
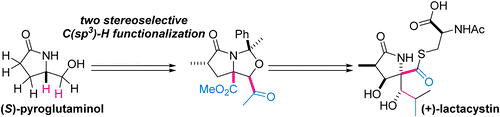
DOI: 10.1021/ol503291s
- M. Nagatomo, H. Nishiyama, H. Fujino, M. Inoue, “Decarbonylative Radical Coupling of α-Aminoacyl Tellurides: Single-Step Preparation of γ-Amino and α,β-Diamino Acids and Rapid Synthesis of Gabapentin and Manzacidin A,” Angew. Chem. Int. Ed. 2015, 54, 1537–1541.
(Selected as Hot Paper)
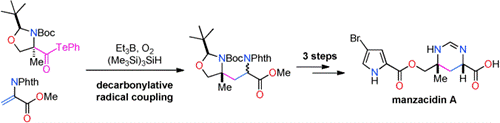
DOI: 10.1002/anie.201410186
- M. Nagatomo, S. Yoshioka, M. Inoue, “Enantioselective Radical Alkynylation of C(sp3)-H Bonds Using Sulfoximine as a Traceless Chiral Auxiliary,” Chem. Asian. J. 2015, 10, 120–123.
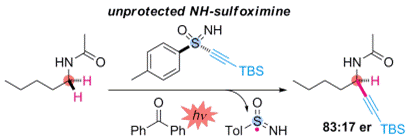
DOI: 10.1002/asia.201402983
- Y. Amaoka, M. Nagatomo, M. Watanabe, K. Tao, S. Kamijo, M. Inoue, “Photochemically Induced Radical Alkenylation of C(sp3)-H Bonds,” Chem. Sci. 2014, 5, 4339–4345.
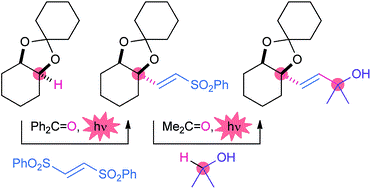
DOI: 10.1039/C4SC01631A
- M. Nagatomo, M. Koshimizu, K. Masuda, T. Tabuchi, D. Urabe, M. Inoue “Total Synthesis of Ryanodol,” J. Am. Chem. Soc. 2014, 136, 5916–5919.
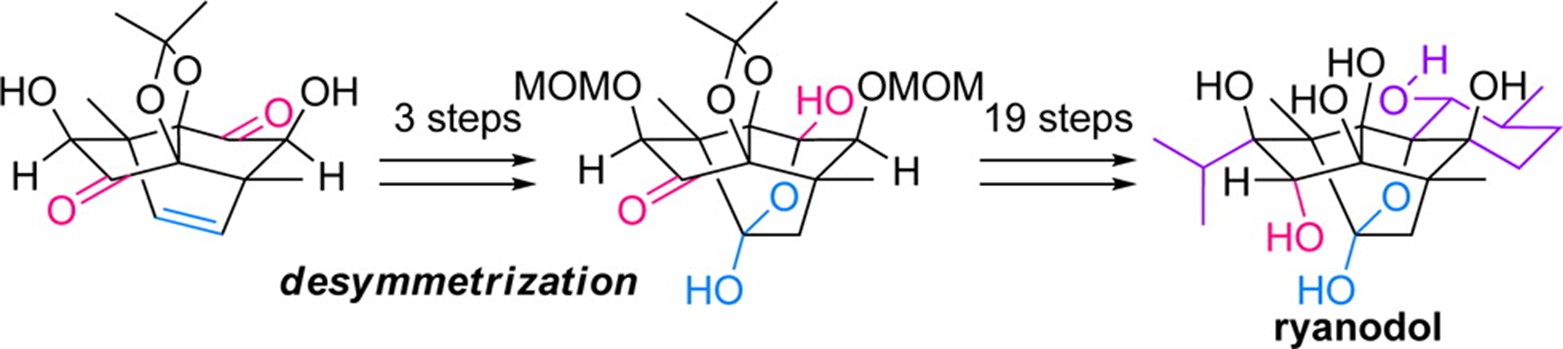
DOI: 10.1021/ja502770n
- D. Kamimura, D. Urabe, M. Nagatomo, M. Inoue, “Et3B‐Mediated Radical-Polar Crossover Reaction for Single-Step Coupling of O,Te-Acetal, α,β-Unsaturated Ketones, and Aldehydes/Ketones,” Org. Lett. 2013, 15, 5122–5125.
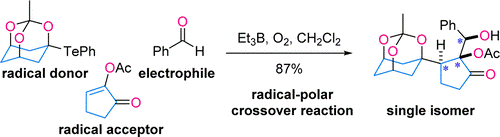
DOI: 10.1021/ol402563v
- Y. Amaoka, M. Nagatomo, M. Inoue, “Metal-Free Fluorination of C(sp3)-H Bonds Using a Catalytic N-Oxyl Radical,” Org. Lett. 2013, 15, 2160–2163.
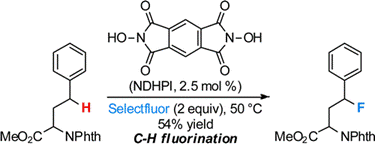
DOI: 10.1021/ol4006757
- D. Urabe, M. Nagatomo, K. Hagiwara, K. Masuda, M. Inoue, “Symmetry-Driven Synthesis of 9-Demethyl-10,15-dideoxyryanodol,” Chem. Sci. 2013, 4, 1615–1619.
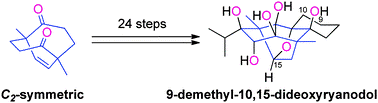
DOI: 10.1039/C3SC00023K
- M. Nagatomo, T. Nakata, “Stereoselective Synthesis of Maitotoxin GHI-Ring System Having a 1,2-Diol Side Chain,” Heterocycles 2008, 76, 1069–1074.
DOI: 10.3987/COM-08-S(N)113
総説
- 彦根悠人、長友優典、井上将行 “複雑分子構築の単純化:レジニフェラトキシンの3つの全合成経路の確立” 有機合成化学協会誌 2023, 81, 1136–1149.
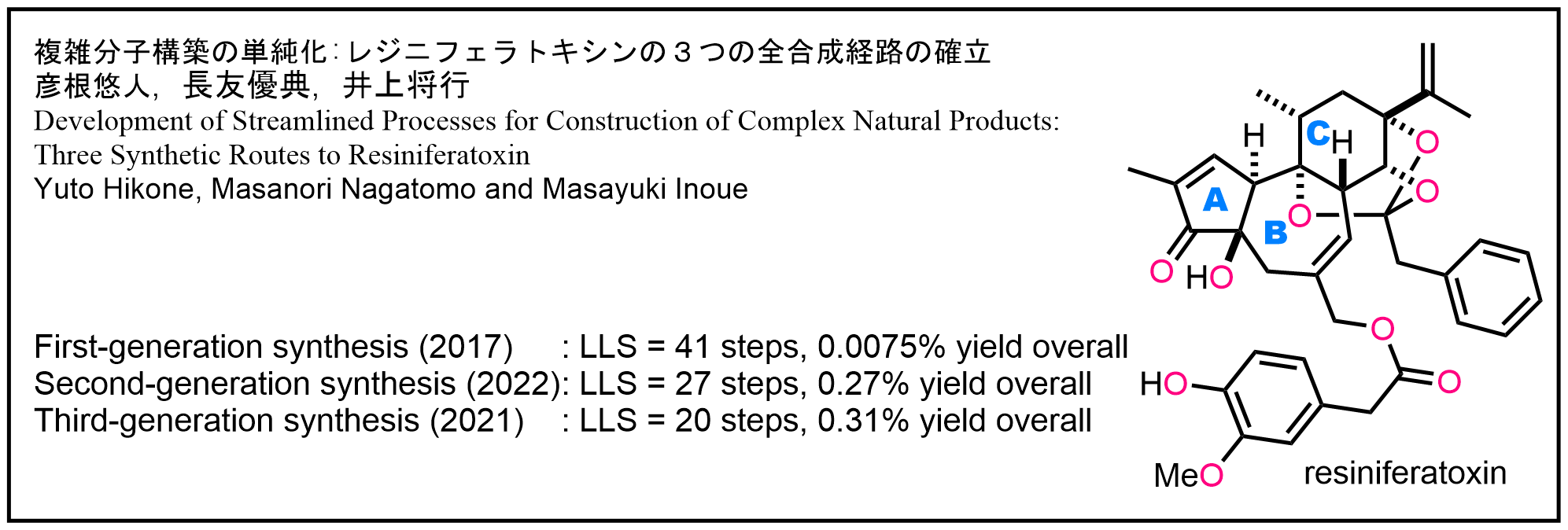
DOI: 10.5059/yukigoseikyokaishi.81.1136
- H. Fujino, M. Nagatomo, M. Inoue, “Total Syntheses of Hikosamine and Hikizimycin,” J. Org. Chem. 2021, 86, 16220–16230.
(Selected as the cover picture link)
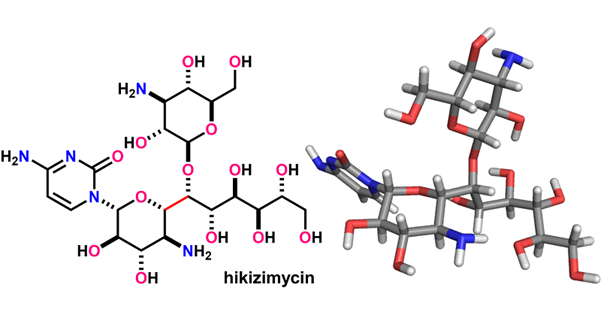
DOI: 10.1021/acs.joc.1c01773
- M. Nagatomo, M. Inoue, “Convergent Assembly of Highly Oxygenated Natural Products Enabled by Intermolecular Radical Reactions,” Acc. Chem. Res. 2021, 54, 595–604.
DOI: 10.1021/acs.accounts.0c00792
- 長友優典、”高酸化度天然物の全合成戦略の開発:光化学的C(sp3)-H官能化を利用したラクタシスチン及びザラゴジン酸Cの全合成,” YAKUGAKU ZASSHI 2019, 139, 651–661.
DOI: 10.1248/yakushi.18-00210
- 長友優典、”窮すれば通ず-リアノジン全合成からの学び,” 有機合成化学協会誌 2018, 76, 494–497.
DOI: 10.5059/yukigoseikyokaishi.76.494
- 長友優典, “オキサジリジンを用いたスチレン類の不斉アミノヒドロキシル化反応,” 有機合成化学協会誌 2013, 71, 944–945.
DOI: 10.5059/yukigoseikyokaishi.71.944
著書
- 長友優典 “網羅的全合成戦略の探求 プリビレッジな構造の抽出と構築,” 化学と工業 飛翔する若手研究者, 日本化学会, pp 584–585 (2023).
- M. Nagatomo, “Total Syntheses of Densely Oxygenated Natural Products by Radical-Based Decarbonylative Convergent Assembly,” New Tide of Natural Product Chemistry, Springer, Singapore, pp 259–273 (2023).
DOI: 10.1007/978-981-99-1714-3_12
- Submitted by Y. Xue, A. Parsad, G. Dong, Checked by J. Han, M. Nagatomo, M. Inoue, “α-Arylation of Cyclopentanones by Palladium/Enamine Cooperative Catalysis,” Org. Synth. 2023, 100, 99–112.
DOI: 10.15227/orgsyn.100.0099
- Submitted by M. Varghese, H. E. Caputo, R. Xiao, A. Balijepalli, A. Hamoud, M. W. Grinstaff, Checked by K. Oga, M. Nagatomo, M. Inoue, “Stereoselective [2+2] Cycloadditions: Synthesis of a Tri-O-Bn-D-Glucal-derived β-Lactam,” Org. Synth. 2021, 98, 491–508.
DOI: 10.15227/orgsyn.098.0491
- Submitted by Y. Chen, Q. Chen, L. Tan, L. Chen, X. Wang, Checked by Y. Komori, M. Nagatomo, M. Inoue, “Preparation of 1H-Indazole-3-carbonitrile,” Org. Synth. 2020, 97, 314–326.
DOI: 10.15227/orgsyn.097.0314
- Submitted by J. Klepp, W. Dillon, Y. Lin, P. Feng, B. W. Greatrex, Checked by Y. Imamura, M. Nagatomo, M. Inoue, “Preparation of (-)-Levoglucosenone from Cellulose Using Sulfuric Acid in Polyethylene Glycol,” Org. Synth. 2020, 97, 38–53.
DOI: 10.15227/orgsyn.097.0038
- Submitted by Y. Kawashima, T. Furukawa, N. Chatani, M. Tobisu, Checked by T. Fukuda, M. Nagatomo, M. Inoue, “Nickel-Catalyzed Cross-Coupling of 2-Methoxynaphthalene with Methyl 4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)benzoate,” Org. Synth. 2019, 96, 36–52.
DOI: 10.15227/orgsyn.096.0036
- 長友優典, 井上将行, “リアノダンジテルペンの統一的全合成,” 天然有機化合物の全合成 独創的なものづくりの反応と戦略(CSJカレントレビュー 27) (日本化学会 編), 化学同人, pp 103–109 (2018).
依頼解説記事
- 長友優典, 今村祐亮, 井上将行, “抗がん剤タキソールの全合成――ラジカル反応を活用した新しい合成戦略とその展開,” 化学 2023, 78 (7), 33–37.
(リンク)
- 長友優典、”高酸化度天然物の全合成戦略の開発,” 薬事日報 2018, 11995, 21.
- 井上将行、 長友優典、占部大介、”ラジカル反応を基盤とした高酸化度天然物の収束的合成戦略,” ファルマシア 2017, 53(9), 860–864.
- 長友優典, 井上将行, “リアノジン類の統一的全合成-多様性を志向した共通中間体の設計戦略,” 化学 2016, 71(4), 47–48.
(リンク)
- T. Hoshikawa, M. Nagatomo, M. Inoue, “Pentafluorophenyl Isocyanate,” e-EROS 2013, RN01621.
DOI: 10.1002/047084289X.rn01621











































